Hydrometers
HYDROMETERS. The first account of the use of hydro meters in England is in a paper published by Robert Boyle in 1675, Phil. Trans., June 1675, and fig. I is reproduced from an illustration of a hydrometer contained in that paper.
The recognition of the fact that such an instrument provides a ready means of de termining the density of a liquid dates from very early times and there is evi dence that Archimedes (287-212 B.C.) was familiar with it. The hydrometer remained of little importance until it was developed for industrial purposes. This development began with the use of hydrometers in the I 8th century for determining the strength of spirits and has gone on until at the pres ent time hydrometers have very wide spread industrial applications.
Boyle's hydrometer (fig. I) was made of glass and differs in no essential feature from modern glass hydrometers (fig. 2). The principle on which the construction of hy drometers is based is that a body floating partially immersed in a liquid is in equili brium when the volume of liquid displaced, which is equal to the volume of the submerged portion of the body, has a mass equal to that of the floating body. When placed in a liquid in which it can float freely a hydrometer will therefore come to rest in such a position that it displaces a volume of liquid hav ing a mass equal to that of the hydrometer. A hydrometer is read in a liquid by noting the intersection of the level liquid surface with the stem of the hydrometer when the hydrometer is at rest and floating freely in the liquid. The reading is taken on a scale sealed inside the stem of the hydrometer.
If V' is the volume of that portion of a hydrometer which is below a particular graduation mark on the scale, then 6V' is the mass of liquid, of density 6, displaced when the hydrometer is floating freely with this graduation in the level of the liquid sur face. If M is the mass of the hydrometer it follows that 6 V' = M and S= M/V'. Consequently if V is the volume of that portion of a hydrometer which is below the lowest graduation mark on the scale and v is the volume of the portion of the stem between the highest and lowest graduation marks the range of densities which can be determined by means of the hydrometer is that lying between the values 8 = M/V -E v and 6=M/V.
A
Logical System of Hydrometry.—Neglecting, for the moment, the comparatively small effects of surface tension and changes of temperature, the plane of intersection of the level of a liquid surface with the stem of a hydrometer is determined solely by the density of the liquid. If, therefore, a scale is sealed inside a hydrometer stem having graduation marks suitably spaced and numbered to enable the density corresponding to any point on the scale to be easily read, the hydrometer will indicate directly the property of the liquid which determines the reading of the hydrometer. Densities are generally most conveniently expressed in terms of grammes per millilitre which is therefore a convenient basis for the density scale of hydrometers.Let us assume, therefore, that we have decided to have our hydrometers provided with scales to indicate densities in grammes per millilitre, this being a basis resting on fundamental units of mass and volume and free from all ambiguity.
The next thing to consider is the practical use of such a hydrometer. In the petroleum industry the density of petroleum products (often expressed as the equivalent specific gravity S6,0 is used as one of the criteria of quality. Since the liquids 6o' F in question have comparatively high coefficients of expansion it is necessary to use the density at some specified temperature as a criterion for comparative purposes. The temperature generally adopted is 6o° F, and a hydrometer adjusted to indicate densities at this temperature will give directly the desired density if read in a sample at 6o° F. It is not always convenient, however, to bring the temperature of the sample to 6o° F, and it is often more economical in expenditure of time to take the hydrometer reading at the prevailing temperature of the sample and to use tables to obtain from the hydrometer reading the density of the sample at 6o° F.
The correction tables can conveniently take the form shown at bottom of page.
Alternatively, to eliminate the need for addition or subtraction, the actual densities at 6o° F corresponding to the observed hydrometer readings can be entered in the body of the table. If this is done, more voluminous tables are required, as the first column should then progress by small increments, e.g., • • • 0.700, 0.701, 0.702 • • . and so on. The combination of hydrometer and tables provides a rapid and reliable means of ascertaining the density of a petroleum spirit at 6o° F from simple observations carried out at any convenient temperature.
As another example we will consider the use of a density hydrometer for ascertaining the percentage composition of sugar solutions. The hydrometer is read in the sugar solution whose temperature is also noted and then by means of tables prepared as indicated below the required percentage of sugar is obtained.

Summarizing the scheme, the hydrometer is used to deter mine density and tables are used to convert the observations to a standard basis, i.e., to percentage composition, etc. The `advan tages of such a system are :—(I) The hydrometer scale is clearly defined and not dependent on the properties of a particular liquid; (a) the onus on the manufacturer is simply to produce accurate density hydrometers ; (3) the conversion of density to percentage composition, etc., is left to the users who should be in the best position to employ the most reliable data relating to the liquids in which they are interested; (4) tables can be revised without rendering any hydrometers obsolete; (5) the hydrometers can be used in a variety of liquids provided there are not too great variations in surface tension.
The general acceptance of the principle that it is the function of the hydrometer to determine density and of tables to inter pret the results would lead to considerable simplification in hydrometry and remove much existing confusion.
Details of Construction of Glass Hydrometers.—Glass hydrometers should be well annealed and made from glass having low thermal hysteresis, resistant to chemical action and free from striae and similar defects. The stem should be cylindrical but considerable latitude is permissible in the shape of the bulb. Some common forms are shown in fig. 2. The hydrometer must be loaded so that it floats with its stem vertical. The loading material, if mercury, should be confined to a lower bulb (figs. 2a, 2d, 2e) ; if lead shot, it should be confined to a lower bulb or fixed in position with wax if placed in the main bulb as in figs.
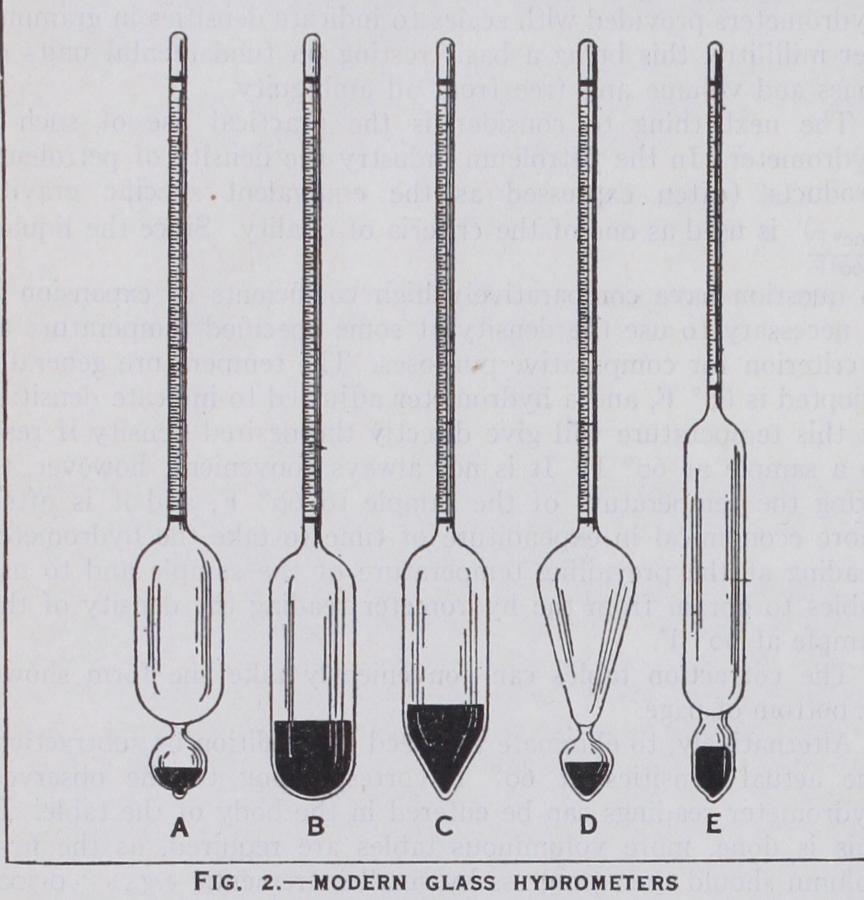
2b and 2c.
Good quality paper should be used for the scale which must be securely fixed in position in the stem and there should be a reference mark etched on the stem coincident with a second reference mark on the paper scale so that any accidental shift of the scale is made evident by a separation of the reference marks.
The graduation marks must be horizontal when the stem is vertical and the scale should be free from twist so that the gradu ation marks lie vertically beneath each other and do not lie on a spiral round the stem. The length of the graduation marks should be suitably varied and sufficient marks numbered to enable the hydrometer to be read at a glance. The distance between adjacent graduation marks should not be less than i mm. nor more than 3 mm.
The scale should bear an inscription indicating precisely the basis of graduation, e.g., "Gms./ml. at 15° C" is a suitable in scription for a hydrometer adjusted to indicate densities in grammes per millilitre at i5° C.
Relation Between Volume of Stem and Volume of Bulb.—Let M=the mass of the hydrometer in grammes.
the density corresponding to the highest graduation mark in gram./ml.
the density corresponding to the lowest graduation mark in gram./ml.
V =volume of portion of hydrometer below the lowest gradu ation mark.
v = volume of stem between highest and lowest graduation marks in ml.
Then we have Spacing of Graduation Marks on Density Hydrometer—Let S gms./ml. be the density corresponding to any graduation mark intermediate between those corresponding to the limiting den sities and and let v' be the volume of the stem between the marks corresponding to the densities and Then we have =S(V+v—v') and by eliminating M and V we obtain v' = v X 6-6° X b If the stem is of uniform diameter and L is the distance be tween the marks corresponding to and and 1 is the distance from the highest graduation mark of the mark corresponding to S then S I=LXS S X So.
0 For a hydrometer of range 1.000 gms./ml. to 1.050 gms./ml. having L = i 5omm. the above relation gives the following values: The length corresponding to a change in density of o•oio gms./ml. therefore decreases towards the lower end of the scale, i.e., the graduation marks on a density hydrometer become more closely spaced towards the lower end of the scale.
Temperature Corrections for Density Hydrometers.— Suppose a density hydrometer, adjusted to indicate densities in gms./ml. correctly at t° C, to read S gms./ml. in a liquid having a temperature t° C and also to read S gms./ml. when placed in a second liquid having a temperature t'°C.
The density of the first liquid is S gms./ml. at t° C where 6=111/V, ill gms. being the mass of the hydrometer and V the volume of the portion of the hydrometer below the mark S.
When the hydrometer is reading S gms./ml. in the second liquid having a temperature t'° C the volume of the submerged portion of the hydrometer is V { i } ml. where a is the coefficient of cubical expansion of the glass from which the hydrometer is made. The density S gms./ml. at t'° C of the second liquid is therefore given by the equation M V{ or to a close approximation a'=8{ I —a(t'—t){ Let c be a correction to be applied to the reading S gms./ml. in the second liquid in order to give the density S' gms./ml. of that liquid at l'° C. Then 6'=S-{-c whence c= Sa • (t — t') .
An average value for a is 0•000026, so that if (t—t') is equal to io°C we obtain the following values of c for various values of S: Now an error of 0.0005 gms./ml. in density is negligible for most purposes for which hydrometers are used and so the above values of c are negligible in magnitude. Hence a density hydrometer which indicates densities correctly in gms./ml. at its standard temperature t° C may be used at any temperature within the range (t± 1o)° C and will still indicate densities at the tem perature of observation with a sufficient degree of accuracy for all ordinary purposes.
Effects of Surface Tension on Hydrometer Readings.— When a hydrometer is floating in a liquid the surface of the liquid does not remain level up to the point of contact with the emergent stem of the hydrometer, but liquid piles up against the stem as shown in fig. 3. There is a down ward pull on the stem of the hydrometer equal to the product of the surface tension of the liquid and the perimeter of the stem, i.e., equal to (TX dynes for a stem of circular cross section where T is the surface tension in dynes per cen timetre and d mm. is the diameter of the stem. The effect of this is virtually to increase the mass of the hydrometer by an amount equal to irdT gms. where log g is the acceleration due to gravity.
Suppose that a hydrometer reads 6 gms./ml. in a liquid having a surface ten sion dynes/cm. and that this reading correctly represents the density of the liquid. Now suppose the hydrometer to be placed in a second liquid having the same density 6 but a greater surface tension T2 dynes/cm. The virtual increase in mass due to surface tension in the second liquid will be greater than that in the first liquid by an amount equal to Hence the hydrometer will sink further in the second liquid than in the first until the increase in the mass of liquid displaced by the hydrometer is equal to the increase in the mass of liquid in the meniscus. If 1 mm. is the additional length of stem sub merged in the second liquid the increase in the mass of liquid displaced by the hydrometer is and 4) represents the error in terms of subdivisions corresponding to a change in surface tension amounting to { T2- T11 . It is obviously desirable that 4 should be as small as possible, i.e., that Xd should be as large as possible. Sufficient attention is not always paid to this fact in the design of hydrometers.
The following example will serve to illustrate the magnitude of surface tension effects. For a hydrometer of range 1 •000 gms./ml. to 1.040 gms./ml. having X =1.5 mm. and equivalent to 0.00i gms./ml. we have if 6= 1.030 gms./ml. and d= 5mm.
4)=0.053 { • If such a hydrometer were to be adjusted to read correctly at 1.030 gms./ml. in dilute sulphuric acid having a surface tension of 7o dynes/cm. and were subsequently to be used in milk of the same density but having a surface tension of 5o dynes/cm. then is 1.03, so that the hydrometer would be in error when used in milk by slightly more than one whole subdivision, i.e., slightly more than o•001 gms./ml.
Some liquids, notably dilute aqueous solutions, have high sur face tensions when the liquid surface is perfectly clean but even very slight contamination may lower the surface tension con siderably, e.g., by as much as 20 dynes/cm. The errors conse quent upon such variations in surface tension are the most serious of the limitations to the accuracy attainable with hydrometers.
Specific Gravity Hydrometers and Density Hydrom eters.—Glass specific gravity hydrometers are very extensively used in many industries and in this country the majority are adjusted to indicate specific gravity S6o°F at 6o° F. If n is a 6o° F reading on such a hydrometer at 60° F the density of the liquid in which the reading is taken is n X o•999041 gms./ml. since the density of water at 6o° F is 0.999041 gms./ml. and specific gravity is simply the ratio :—density of liquid at : density tz° of water at The scale of a specific gravity hydrometer more closely spaced towards the bottom exactly as on a hydrometer, in fact, a hydrometer indicating at 6o° F 6o° F could be converted to indicate densities in grammes per milli litre at 6o° F simply by increasing its mass in the ratio 1: Though the basis given is that most commonly employed, many others are also used, e.g., c at 15° C, c at 17.5° C, 15°C 17.5° C c at 20° C and so on.
20° C Hydrometers adjusted to indicate densities in grammes per millilitre are preferable from a scientific point of view to hydrom eters indicating specific gravity, because their scales are more directly related to the fundamental units of measurement. They are not so widely used as specific gravity hydrometers but they are coming into more general use.
Hydrometers for Ascertaining the Strength of Alcohol.
History of Development in Great Britain.—Hydrometers came into use for ascertaining the strength of spirits towards the end of the 17th century, when they began to displace such compara tively crude tests as the well-known powder test. In the powder test spirit was poured on to a small quantity of gunpowder and then a light was applied to the wet powder. If it burned the spirit was "over-proof" and if not the spirit was "under-proof." The earliest hydrometers resembled that shown in fig. 1 and had a mark near the middle of the stem at which the hydrometer would float in a mixture of about equal parts of alcohol and water. A mark at the bottom of the stem indicated the reading in water and one at the top the reading in the strongest alcohol then known. If the middle mark came well below the surface of a sample of spirit the spirit was "over-proof." This form of hydrometer gave some degree of precision to the term "proof spirit" but only roughly indicated "over" and "under-proof" strengths. With the increase in duties and the increased need for more accurate discrimination between the strengths of different spirits considerable development of hydrometers occurred in the 18th century, more closely divided scales were used, and metal hydrometers with detachable weights of various sizes were made. It was not, however, until quite the end of the 18th century that the necessary data for placing alcoholometry on a sound basis was available.In 1794 George Gilpin, clerk to the Royal Society, published a comprehensive set of tables based on a long series of determina tions of the specific gravity of mixtures of alcohol and water.
These tables gave the specific gravity I. for each degree Fahren 6o F heit from t =3o° F to t =8o° F, of 201 different mixtures of alcohol water, the strength of the mixtures being expressed thus:— The tables also gave the percentage composition by volume of each mixture and by 1 794 all the necessary data existed for obtaining the strength of any sample of spirit from a determination of its density.
The problem remained, however, of linking up the available data with the simplest method of determining density, i.e., by means of a hydrometer.
In 1802 the Government asked for hy drometers to be submitted to them for examination with a view to the adoption for revenue purposes of the one considered most suitable for determining the strength of spirits. As a result a hydrometer and tables submitted by Bartholomew Sikes were adopted for revenue purposes and legalized by Acts of Parliament of 1816 and 1818. In all its essentials Sikes's sys tem remains in use at the present time.
The outstanding features of Sikes's pro posals were : (1) he gave precision to the then customary method of expressing strength in terms of "proof-spirit"; (2) he appreciated the desirability of keeping the hydrometer as simple as possible and the necessity for providing tables to obtain adequate accuracy in determining proof-strength from hydrometer readings; (3) he prepared his tables accurately and in a form convenient for use.
Proof spirit was defined (56 Geo. III. c. 140, 1816) as "that which at the temperature of 5 r ° F weighs exactly 4 parts of an equal measure of distilled water." Proof spirit so defined con tains 49.28% by weight of pure anhydrous alcohol. If from of a given spirit containing more alcohol than an equal volume of proof spirit, 12ogals. of proof spirit could be obtained by the addition of water, the spirit was said to be 2o% over proof, and ioogals. of that spirit would be charged the same duty as 12ogals. of proof spirit. Similarly a spirit Ioogals. of which contained the same amount of alcohol as 8ogals. proof spirit was 2o% under-proof and dutiable at 8o% of the rate fixed for proof spirit. On this basis it is only necessary to fix the amount of duty payable on one gallon of proof spirit and the duty chargeable on any particular quantity of spirit can then be reckoned from its bulk and proof strength. By his hydrometer and tables, Sikes provided a ready means of ascertaining proof strengths.
Sikes's Hydrometer.—The Sikes hydrometer in use at the present time is shown in fig. 4. It is a gold-plated brass hydrom eter with a spherical bulb about 4cm. in diameter. Above the bulb is a hollow rectangular stem about 9.5cm. in length and 5mm. X 2mm. in cross section. On this stem a scale about 6.5cm. in length and subdivided into so equal parts is engraved. The highest graduation mark is carried completely across the face of the stem and numbered "o," the next four graduation marks are shorter lines, the fifth line is carried completely across the stem and numbered "1" and so on down to the lowest mark which is numbered "1o." In the original hydrometer there were no sub divisions between the graduation marks. Below the bulb is a stem about 3cm. in length which is circular in cross-section, tapers towards the bulb and has a balancing poise fixed at its lower end.
There are a series of nine gold-plated brass weights for use with the hydrometer. Each weight is in the form of a disc with a central hole and slot cut in it as shown in fig. 5. The size of the slot is such that it will easily pass over the upper end of the lower stem of the hydrometer but will not pass over the thicker lower end of this stem. The central hole is large enough to allow the weight to be placed on the lower stem and rest on the top of the balancing poise. The weights are graded in size and num bered 10, 20, 3o and so on up to 9o. They are adjusted so that if the hydrometer reading in a suitable sample of spirit is ro on the scale when the 20 weight is attached to the hydrometer, then the scale reading in the same sample of spirit when the 20 weight is replaced by the 3o weight will be o. The hydrometer reading in any sample of spirit is the sum of the number marked on the weight required to make the hy drometer float with part of its stem sub merged in the liquid and the scale reading at the intersection of the level liquid sur face with the stem. The complete range of readings is thus o to ioo, since the hy drometer may be used alone or with any one of the nine weights placed on the lower stem.
Tables for Use with Sikes's Hy
drometer.—The present tables for use with Sikes's hydrometer differ from the original ones only in that they cover a larger range of temperature, progress by fifths of a degree Sikes instead of whole degrees, and are based on later determinations of the density of alcohol-water mixtures. A portion of the table for 6o° Fahrenheit is given below :- The table for 6o° F extends over the whole range from o to 100 on the Sikes scale in steps of 0.2 as above and there is a similar table for each degree Fahrenheit from 3o° F to Ioo° F inclusive. To ascertain the strength of a sample of spirit all that is necessary is to read the hydrometer in the spirit, observe the temperature of the sample at the time the reading is taken, and then refer to the table for the particular temperature observed in which the required proof strength will be found against the observed hydrometer reading.
Extension of Range.
Sikes's hydrometer instrument, used without any weight attached, sinks below the o mark at 3o° F in spirits containing more than 97.1% by weight of alcohol (72.2% O.P.) and at Ioo° F in spirits containing more than as little as 85.5% by weight of alcohol (57.5% 0.P.). For strong spirits an additional hydrometer, similar to the Sikes hydrometer, and having a scale numbered "A" o to "A'' io has therefore been introduced for revenue purposes in Great Britain. The hydrometer reads "A" io, the lowest mark on the stem, in a spirit in which the ordinary range Sikes hydrometer reads o. In India a hydrometer of range "A" o to "A" 20 is used for strong spirits, "A" 20 on this scale being equivalent to o on the ordinary Sikes scale, and "A" io being equivalent to "A" o on the English scale for strong spirits. Such complications seem inevitably to follow the adoption of arbitrary scales.Alcohol Hydrometry in Other Countries.—Hydrometers and appropriate tables are widely used for ascertaining the strength of spirits. (For details see Alcoholometric Tables by Sir Edward Thorpe.) A proof standard similar to the English is used in America and Holland but both differ from the English and also from each other. In Russia, France and Italy the strength of spirits is expressed as percentage of alcohol by volume, and in Germany as percentage of alcohol by weight. There is even greater diversity in the hydrometers employed. There are purely arbitrary scales like the Sikes ; in Russia a hydrometer indicating Ioo in water, and o in strong spirits, is used and in Holland a scale of 28 degrees each representing an equal length on the scale is used. Other hydrometers in use are adjusted to indicate per centage of alcohol, some by weight, some by volume, and still other hydrometers have scales indicating proof strengths.
Since the density of a spirit serves as a measure of its strength and since also its density determines the position of equilibrium of any hydrometer placed in the spirit it is obviously possible to correlate any hydrometer scale and spirit strengths. The same thing applies to other liquids and it is therefore easy to under stand how a large variety of hydrometers has come into use not only in alcoholometry but for other purposes also. The result is obviously not without its disadvantages.
Saccharometers.—Bates saccharometers are used for esti mating the duty on the sugar content of worts, the liquid from which beer is produced by fermentation. The duty is reckoned on the basis of the specific gravity F) of the worts and the 60°F // Bates saccharometer is a gold-plated metal hydrometer adjusted to indicate specific gravity F\ when read in worts at 60° F. 60°F/ It has a rectangular stem on which a scale numbered o to 3o engraved, this range being equivalent to a change in specific gravity of 0.030. The scale is divided into 3o intervals, each corresponding to o•oo1 change in specific gravity. A series of weights numbered 1,000, 1,030, 1,060 and so on are used with the hydrometer. They are olive-shaped and each is provided with a conical stem which is a tight fit in a conical hole in a vertical ring fixed to a short stem below the hydrometer bulb (see fig. 6). With the i,000 weight attached the range of the hydrometer is from S&P F 1.000 to Soo° F 6o° F 6o° F 1.030 with the 1,030 weight S60° F 1.030 6o° F to 1.06o and so on. In order that the same scale may be used with each weight it is necessary not only that the mass of the weights must be adjusted correctly but also the volume of each weight must be greater than that of the next smaller weight by an amount equal to the volume of the stem between o and 3o.
Glass hydrometers adjusted to indicate density and also those adjusted to indicate specific gravity are extensively used both in the brewing and the sugar industries.
The Brix saccharometer simply indicates percentage of sugar by weight when read in sugar solutions at 17.5° C.
Some glass hydrometers are scaled so that when read in hot sugar solutions, e.g., at 150° F, they indicate the specific gravity F which the solution would have if cooled down to 6o° F.
6o° F
Other hydrometers have an enclosed thermometer but the thermometer scale indicates not temperature but corrections to be applied to observed readings on the hydrometer scale due to any difference between the temperature of the solution and that at which the hydrometer scale is correct.The last two types of hydrometer are not to be recommended. It is a mistake to try to make a hydrometer perform the function of both hydrometer and correction tables.
Twaddle Hydrometers
are generally made of glass and adjusted for use at 6o° F. The Twaddle scale is defined by the relation 200+n =S or 200 where n is the scale reading at 6o° F in degrees Twaddle and s is the corresponding specific gravity S60° F.
60° F
A complete set of Twaddle hydrometers consists of six hydrom eters having the ranges o°-24° ; 24°-48° ; 48°-74° ; 1o2°-138°, 138° to 17o° respectively. The complete range o° to 170° (i.e., Soo°F 1.000 to See F 1.850) covers the range of 6o° F 6o° F specific gravities of mixtures of sulphuric acid and water from that of pure water to that of pure acid. The hydrometer scales in hydrometers forming a set like the above are usually subdivided into intervals representing o.5° on the Twaddle scale.The above ranges are those most frequently met with, but Twaddle hydrometers are also made in a variety of other ranges and with various degrees of openness of scale.
It is clear from the relation defining the Twaddle scale that a change in reading of one degree on a Twaddle hydrometer repre sents a change of 0.005 in specific gravity. Hence a specific gravity hydrometer indicating Soo. F at 6o° F and subdivided into 6o° F intervals representing 0.005 change in specific gravity could be converted into a Twaddle hydrometer simply by re-numbering the scale. The only apparent reason for having Twaddle hydrom eters as well as specific gravity hydrometers is that they give a scale confined to three figure numbers, and when an accuracy of ° Twaddle suffices avoid the use of a decimal point. Twaddle hydrometers serve no purpose which could not equally well be fulfilled by either density or specific gravity hydrometers.
Baume Hydrometer.
In 1768 Antoine Baum published in L' Avant directions for making the hydrometers which are known by his name. The hydrometer for use in liquids heavier than water was to read o° Be in water at a temperature of 1o° Reaumur and 15° Be in a 15% salt solution at the same temperature. The scale between o° Be and 15° Be was to be divided into 15 divisions each equal in length, and prolonged by similarly spaced graduation marks beyond the 15° Be mark. The hydrometer for use in liquids lighter than water was to read o° Be in a 1o% salt solution at 1o° Reaumur and 1o° Be in water at the same temperature, the scale being prolonged upwards above the water point by equally spaced divisions each equal in length to one-tenth the distance between the o° Be and 1o° Be marks.The Baum hydrometer appeared at a time when other hydrom eters were becoming unduly complicated. Clarke's hydrometer, for example, which was used for spirit assaying prior to the introduction of Sikes's hydrometer, had become encumbered with 54 weights. The Baum hydrometer, with its evenly spaced scale, was easy to manufacture and simple to use. It is not surprising, therefore, that it came into general use, for the scale readings, although in a sense entirely arbitrary, could, if necessary, be correlated with density or any property varying with density.
However, in course of time differences became noticeable when Baum hydrometers from different sources were compared. It may be shown that the readings on any hydrometer having an evenly spaced scale can be expressed by a relation of the type where d is the density corresponding to the reading n and A and B are constants. Consequently, in order to provide greater pre cision in definition of the scale and to preserve the continuity of scales in current use, formulae of the above type were introduced. Unf ortunately, however, no one formula was universally adopted— f ormulae were based on particular instruments and a variety of different ones came into use. In Germany, for example, an inquiry in 1892 showed that there were three different formulae in com mon use. Consequently the formula was proposed, which made the reading o° Be in water at 15° C on both the heavy and light hydrometers, the — sign being used for the heavy hydrometers and the -E- sign for the light ones. This relation, it was proposed, should supersede the three others in common use and later, in 1904, it was adopted as the basis of the official testing of Baume hydrometers by the Kaiserliche Normal-Eichungs-Kommission. However, the three original formulae have survived alongside the new one so that the attempt at standardization has merely resulted in Germany having four different Baume scales in 1928 as compared with three in 1892. Again, in America, the Bureau of Standards about 1904 adopted the formulae for the heavy and light scales as representing the then customary practice in America. In June 1916, however, the Bureau of Standards found it necessary to protest against the use in America of the formula for the light Baume hydrometer. Yet in the Bureau of Standards agreed to accept this basis for light Baume hydrometers for use in the petroleum industry, the scale so defined to be known as the A.P.I. scale, but retained its original formula for light Baume hydrometers for use in liquids other than petroleum (The Bureau of Standards Circular J9, 1916, and Circular 1924)• So much for modern attempts to standardize the Baume hy drometer, but it should also be noted that already in 1881 Prof. Chandler had collected 23 different formulae, proposed at one time or another, for the heavy Baume hydrometer and II for the light hydrometer. There is only one practical way of ending the confusion which has grown up around the Baume hydrometer. This is to discontinue its use entirely and to substitute hydrom eters indicating density directly. (See Glas and Apparat, 1928, IX.-23.) Constant Displacement Hydrometers.—In all the hydrom eters considered so far the variation in displacement—i.e., volume submerged—of a hydrometer of constant mass has served as a means of determining density. It is obviously possible to de termine densities by using a hydrometer of known mass and determining the additional mass necessary to immerse the hy drometer to a single fixed mark corresponding to a known dis placement. Hydrometers, of which the best known is Nicholson's, based on this principle, have been made but they are not so convenient to use as ordinary hydrometers and are of little practical importance.
Relative Merits of Metal and Glass Hydrometers.—The dis advantages of metal hydrometers are :—(1) They are liable to change in weight due to corrosion and wear; (2) the stem is not so readily wetted as the stem of a glass hydrometer; (3) the joints are apt to develop leaks; (4) the bulbs are necessarily made of thin metal and are very liable to become dented. It will be realized that through wear, slight leakage, or a small dent, a metal hydrometer may become seriously in error without this being suspected unless the accuracy of the hydrometer is checked at frequent intervals. A glass hydrometer is free from such dis advantages and provides a much more reliable instrument than a metal one. It is more liable to breakage than a metal hydrom eter, but once broken the damage is apparent and there is no risk of using a hydrometer which has become seriously out of ad justment, which is one of the most serious objections to a metal hydrometer.
Standardization of Hydrometers.
Since the readings on any hydrometer scale can be converted into equivalent densities, the errors of a hydrometer may obviously be obtained by reading the hydrometer in a liquid of known density. It is more con venient, for example, to determine the errors of a hydrometer indicating percentages of sugar by weight, by determining the densities corresponding to various points on the scale rather than by making up a series of sugar solutions to definite concentrations and reading the hydrometer in them.The best method is to determine the density of the liquid and to observe simultaneously the reading of the hydrometer in the liquid. This can be done most conveniently by employing the sinker method of determining density. (See DENSITY.) The hydrometer and sinker can be placed side by side in the liquid and the hydrometer reading observed under precisely the same condi tions as those under which the density is determined. Thorough stirring immediately previous to the observations and efficient temperature control are essential to secure uniform density throughout the liquid, and to bring the temperature of the liquid to that at which the corrections to the hydrometer are required.
The direct standardization of hydrometers by the above method takes considerable time and is generally only employed for hy drometers intended for use as standards for verifying other hydrometers. Given a hydrometer whose scale errors are known, a similar hydrometer may be verified by floating it side by side with the standard and taking simultaneous readings on both hydrometers. The difference between the readings combined with the known error of the standard gives the correction to the scale of the hydrometer under test. If the two hydrometers differ ap preciably in dimensions, due allowance must be made for surface tension effects unless they are compared in the same liquid as that in which the hydrometer under test is to be used and the correc tions to the standard in this liquid are known.
The extensive use of hydrometers in industry and the necessity for accuracy in construction has led to arrangements being made whereby hydrometers may be submitted for verification to national institutions—in England to the National Physical Laboratory, in America to the Bureau of Standards, Washington, in France to the Laboratoire d'Essais, Paris, and in Germany to the Physikalische Technische Reichsanstalt, Charlottenburg.
