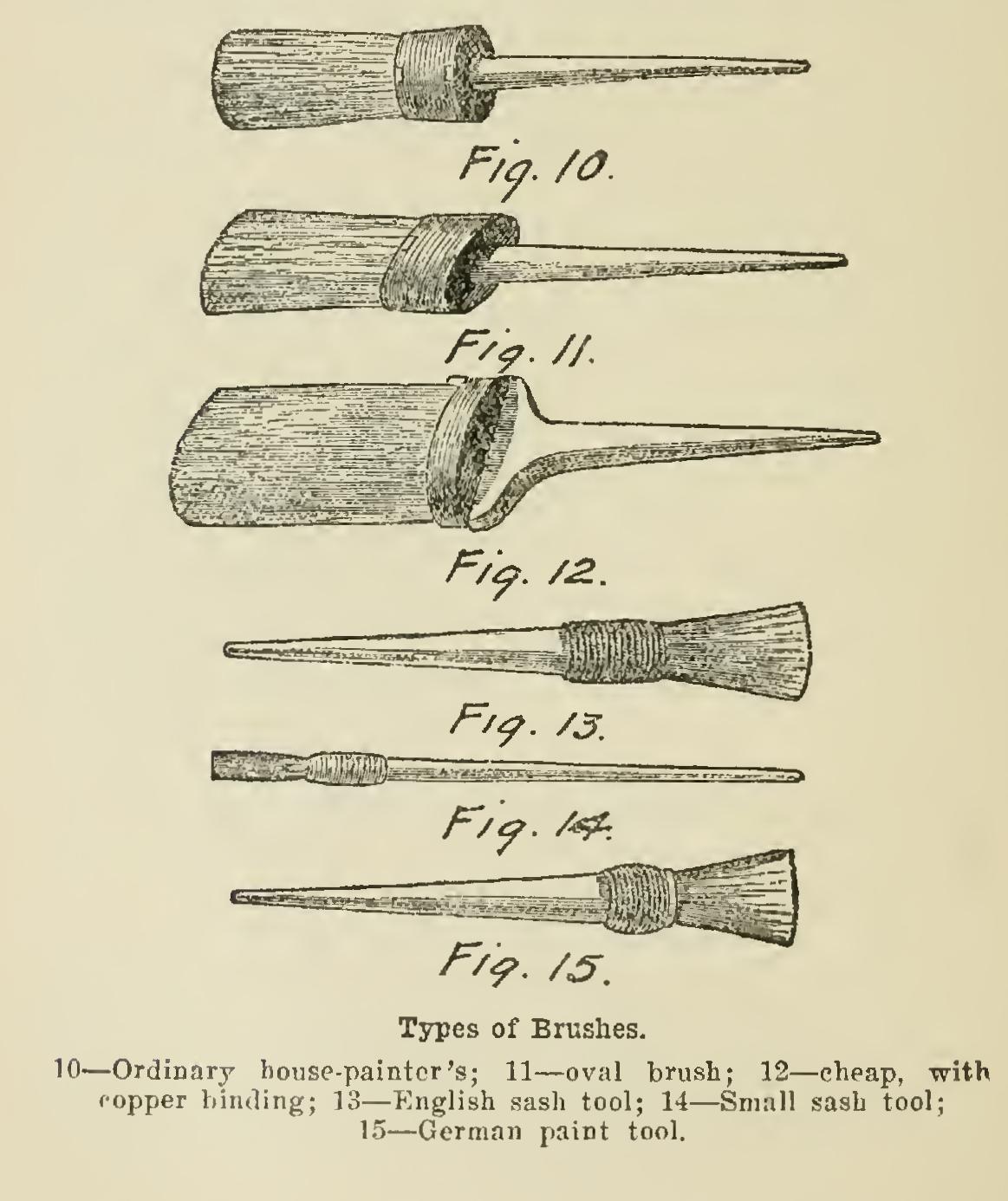
Driers Oils
oil, turpentine, drying, lead, white, color, terebine, pure and pound
Red Lead is a good natural drier, but this would not do for assisting the white paint to oxidize. The principal drying materials for all liquids and paints are sugar of lead, sulphate of zinc, litharge or oxide of lead, white copperas, white sulphate of manganese, and white borate of manganese, of which the two last are the most expensive. Nowadays it is not necessary to rub up our own litharge or sugar of lead, for they are offered to us in the less pure, perhaps, but far more convenient form of liquid driers and patent driers respectively.
Don't use driers which form a dark matter on the top in the keg, nor such as have a brittle skin—for they dry too hard—nor such as turn a livid white under water. Don't use driers which have a body or are of a dark color.
Liquid driers, such as terebine, are composed of oils or spirits which have been subjected to the action of a siccative or drying material. This action of drying is an instance of a quick way of bringing about what nature accomplishes in a longer time. Instead of losing a portion of their bulk, as is the case with water-colors, oil colors, in drying, take up oxygen from the air, a process which chemistry shows is constantly going on with nearly everything on the earth's surface. It is easily seen that the slow drying of nature is the best, and that excess of driers will harden the surface of the color too quickly, and cause the outer surface to contract and become smaller than the under surface. The tension between them causes cracking. It is purposely done to produce fancy cracking in pottery work. The glaze and color are so made that the glaze contracts more quickly than the color in the cooling; and the result is cracks all over the ware. Such substances as Japanese gold size or varnish will make the paint brittle and produce the same effect. Drying oil or sugar of lead is the best to use. Good terebine also is a safe drier.
Every color and varnish manufacturer now makes a terebine or liquid drier, and its drying strength is usually that of one ounce to the pound of paint, under favorable conditions. Terebine combines far more satisfactorily with linseed oil for drying than does Japan gold size, a similar mixture. The latter is most useful in compounding flatting and quick-drying paints for varnishing upon.
A sample of terebine, purchased at a paint shop and afterwards tested, was found to con tain 75 per cent of turps, and 25 per cent of a residue consisting almost entirely of boiled lin seed oil, but containing also a little common resin—added, no doubt, as an adulterant. The boiled oil it contained was of a dark brown color, and had been boiled with oxide of lead (litharge) in order to render it quicker as a drying agent. It is safe to say that terebine may be made by mixing together 25 per cent of boiled linseed oil (treated with litharge) and 75 per cent of turpentine—in other words, three quarters pound of turps and one-quarter pound of oil.
Another recipe for making this useful ma terial for painters is as follows :—Take two pounds ground litharge, two pounds red lead, one pound sulphate of manganese, one-half pound sugar of lead; put all these into a pot, and mix with them four or five gills of pale Japan gold size, until the lot can be easily stirred. Then add about one-half gallon of tur pentine. Now leave the whole to stand, with occasional stirring, for a few days (not less than three), and its materials will act without heat. After the expiration of a few days, the clear resultant liquid—terebine—may be taken off, and more "turps" put on the materials, for the second and third time, with equally satisfac tory results. Ilalf proportions of these com ponent articles may be used with equally good results.
Many samples of paraffin give what is called a is, though the oil is appar ently colorless, if you hold some in a test-tube against a black background (a coat-sleeve), and allow the light to be reflected from it, you will find that it has a fine, delicate blue color or bloom. Now, pure turpentine does not show a fluorescence, and by trial it has been found that 10 per cent of paraffin oil added gave a slight fluorescence on the surface of the mixture. The only point against this test is that some samples of paraffin oil do not give a fluorescence, but there are not many of them; resin oil, the chief adulterant for turpentine, is also fluorescent, and has to be detected by its high gravity. If there is a smell of paraffin oil and fluorescence, it is certain there is paraffin present.
One of the simplest tests is to put a few drops of the turpentine on a sheet of white writing-paper. If the turpentine is pure, it will evaporate in a few minutes, leaving the paper quite clean; while if it be adulterated with par affin oil (petroleum), it will leave a greasy mark. If the adulterant used is benzine, the greasy mark will not be so apparent, and will fade away in the course of about five minutes. Another good test for the practical painter is to shake up the turpentine in a small bottle. It is well to obtain a sample of turpentine of un doubted purity, and place this into a small bottle. Shake up the sample, and note the re suit; then immediately shake up the suspected turpentine, comparing the two. The bubbles in turpentine adulterated with petroleum will hold longer than those in pure material, and a blue cast will be observable if the bottle be held in a strong light. It should be added that old tur pentine that has become gummy, even if pure, will leave a greasy mark on white paper, which might be taken to indicate the presence of pe troleum in the test above mentioned. As, how ever, gum in turpentine is just as objectionable as the presence of pariffin oil, this fact does not interfere with the usefulness of the test.