Determination of Hydrogen-Ion Concentration Electrometric Methods
DETERMINATION OF HYDROGEN-ION CONCENTRATION ELECTROMETRIC METHODS The use of an electrometric method, consisting of the measure ment of an electrical potential, for the determination of hydrogen ion concentrations was first made possible by means of a relation established by Nernst, which exists between the solution pressure of an element when surrounded by a liquid and the osmotic pres sure of the ions already in solution. (See SOLUTIONS.) According to this theorem an element, such as a metal, when in contact with an ionizing medium, such as water, has a definite tendency to pass into solution in the form of ions or electrically charged particles. When the ions carry a positive charge, an equivalent negative charge remains on the surface of the element. An electrical double layer is consequently formed between the surface of the metal and the oppositely charged free ions in the solution. The process of ionic solution will proceed until the potential difference in this double layer reaches a certain critical value which is determined by the specific character of the metal. Further ionization is then arrested by the electrostatic charge of the ions in solution, and the potential difference between metal and solution gives a measure of the ionizing tendency. (See ELECTROLYSIS.) It should be noted that the amount of metal which passes into solution to produce this equilibrium condition is ordinarily below the limits of the amount that can be detected by chemical means. If the metallic ions are already present in the solution in the form of dissociated salt, further ionization of the metal is opposed not only by the electrostatic layer but also by the os motic pressure of the ions already present. In this case metal will dissolve or ionize until the potential of the electrostatic layer formed equals the excess of its solution pressure above the os motic pressure of the ions. With metals such as copper and the noble metals which normally acquire a positive charge in contact with an electrolyte, the solution pressure of the metal is so low that it is below the osmotic pressure of the ions given by the smallest trace of salt of that metal in solution. With these metals deposition occurs of metal ions present in the solution, whereby the electrode becomes positively and the electrolyte negatively charged, until the potential difference balances the excess of osmotic pressure above the solution pressure. If an electrode of platinum or other noble metal be surrounded in its upper part by hydrogen, while the lower part is immersed in an electrolyte, the hydrogen which dissolves to a certain degree in the metal will tend to ionize and pass into the solution as hydrogen ions. A definite potential is thus produced which is determined solely by the pressure of hydrogen in the surrounding atmosphere and by the concentration of hydrogen ions already in solution.
General Theory of the Hydrogen Electrode.
The first amounts of hydrogen are dissolved by platinum according to the distribution law that would prevail if the dissolved gases were in the atomic state, i.e., V p/c' = constant, where c is the concen tration of gas in the metal and p its pressure in the surrounding atmosphere. The quick saturation of the surface is followed by a slow diffusion into the interior. Palladium, which dissolves 500 times to Boo times its volume of hydrogen, is not very suitable for a hydrogen electrode, probably because the high solubility, conjoined with a high rate or diffusion, makes it impossible to set up a quick surface saturation with small quantities of hydrogen. Platinum, which dissolves one to ten times its volume, is the most satisfactory. The surface is greatly increased and its adsorptive capacity thereby raised by platinizing, or covering with finely divided platinum. Gold, which dissolves about half its volume, probably fails owing to its having too low an adsorptive capacity. It is, however, suitable for underlying material ; gold or gold plated electrodes, afterwards platinized, iridized or palladized, have been found satisfactory. The reaction occurring between the platinum and the gaseous hydrogen may be represented by the equation and that between the platinum and the elec trolyte by the equation H±H'. In order to obtain quick satura tion of the electrode, it is necessary that much of this should be in contact with the gas phase.According to the relation derived by Nernst, the potential E between two hydrogen electrodes immersed in solutions of hy drion activities or concentrations and is proved to be E= R T h nF where R= gas constant =8.313 joules, T = absolute temperature, n = valency of ion and F= the faraday = 96, 50o coulombs. Thus, E=0•0001984 T log and at T=273°, 291° and 298° respectively the factor 0.0577 and 0.0S91. The activity of hydrion from a "strong" acid such as HC1 is very nearly equal to the total concentration, if this is low.
The normal potential on the hydrogen scale is defined as the potential of a hydrogen electrode in a solution of hydrion concen tration measured against a hydrogen electrode in a solution of normal hydrion concentration or Ii = r, that is : At 18° C in practice measurements are usually made against another standard half-cell usually the saturated or normal KC1 calomel electrode, the absolute potentials of which have been more or less closely defined against various kinds of null potential electrodes. Sorensen measured the e.m.f. of the combination o•1 HCI I I KCI, at 18° C with diffusion potential eliminated. Assuming that the HC1 is 91•7% dissociated, it is calculated that the potential of the normal hydrion hydrogen electrode against the decinormal KC1 calomel is 0•3380 volt (calomel positive). If the e.m.f. of a hydrogen electrode against the decinormal KC1-calomel electrode is E,, then Ez—o•338 at 18° C.
pH 0.0577 With a saturated KC1-calomel cell, the potential of the electrode with normal hydrion concentration has been as 0.2503 volt at 18° C. Consequently with this standard, we have 250 = PH (Cf. E. B. R. Prideaux, I. Sci. Instr., 1924-25, ii., pp. 33 and 113.) Experimental.—The hydrogen employed must be free from impurities, since compounds such as hydrogen sulphide or arse nide, which may be present in the gas prepared from ordinary zinc and acid, have a poisoning action on the platinum. The hydrogen is most suitably obtained from an electrolytic generator or from cylinders of the compressed gas. The potential of a hydrogen electrode against its solution becomes more positive with decrease in the pressure of the hydrogen (or of its partial pressure in a mixture of gases). The magnitude of the correction to be applied may be calculated by considering the maximum work involved in the transfer of mol. of hydrogen through atoms and ions from to p2; this is dE=RT/2F in p atmospheres. If hydrogen is saturated with water vapour of vapour pressure p' in mm., the total pressure being p, the correction becomes log ,• 76o With an enclosed electrode of the continuous hydrogen type, the general arrangement of the electrodes is shown in fig. I.
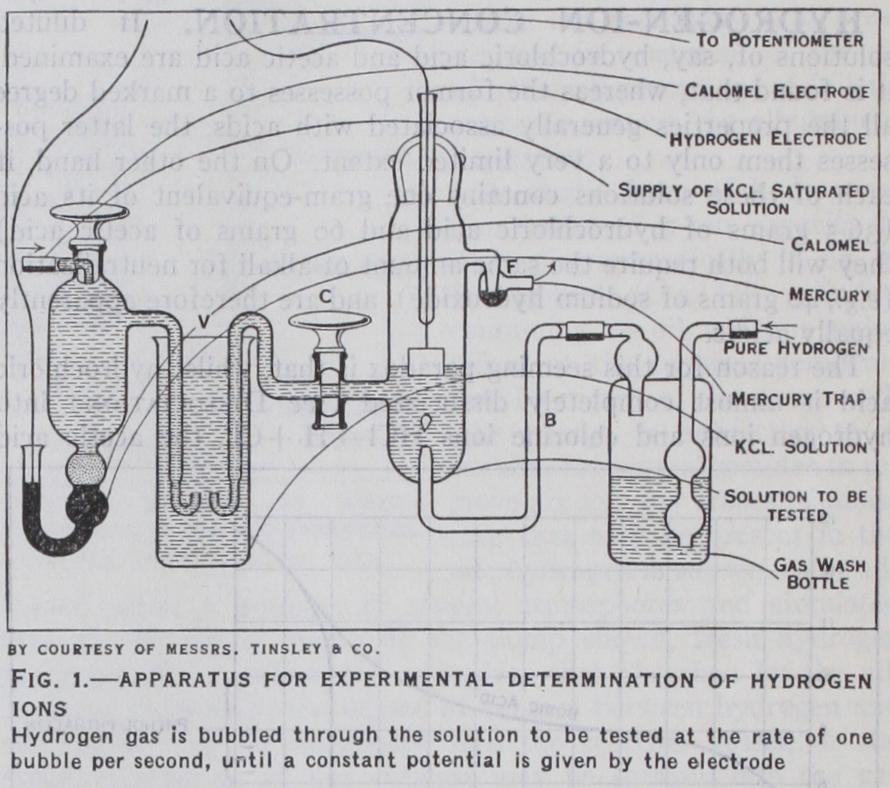
A strip of platinum foil is welded to a short length of platinum wire which is then sealed into the glass tube and makes contact with a drop of mercury inside, or preferably, a copper wire is fused or soldered on this end. To prepare for use the electrodes are first cleaned by immersion in hot chromic acid for five to ten minutes and then well washed with distilled water. They are then coated electrolytically with "platinum black," by sur rounding with an electrolyte consisting of Platinum chloride . . . 3 grams Lead acetate . . . . . . . . 0.02 to 0.03 grams Distilled water . . . . . . . ioo cu.cm.
and electrolysing with the current from a 2 volt accumulator, so that a moderate evolution of gas occurs. The current is reversed every half minute for 15 minutes or longer. The electrodes are then placed in a solution of ferrous and ferric salts acidified with sulphuric acid, in order to eliminate occluded chlorine, and the electrodes are thoroughly washed with distilled water (they must always be kept in distilled water when not in use, as drying spoils the platinum black) . To prepare for measurement the hydrogen electrode vessel is filled with the solution to be tested keeping C closed. In order to saturate with water vapour the gas is passed through a wash bottle filled with the same solution and connected with the rubber tubing to the bent tube B on the electrode vessel.
The gas, if not electrolytically generated, must first be passed through wash bottles containing potassium permanganate solution, and then through a saturated solution of mercuric chloride before passing through the electrode vessel through the tube B. C is opened and some of the solution displaced until it reaches the end of the side tube. C is then closed. A saturated calomel electrode is most conveniently used as the second electrode, and saturated potassium chloride for making the junction. The side tubes of the two electrodes dip into a vessel V containing saturated po tassium chloride. Hydrogen is passed through the hydrogen elec trode at the rate of a bubble per second for about three hours, escaping through the trap F, until absorption by the platinum ceases and a constant potential is given by the electrode. Modi fications in the type of electrode employed have been applied for special purposes. Point electrodes are used for giving quick read ings with a fixed volume of hydrogen without stirring or shaking, and in cases where only small volumes of solution are available. The use of an oscillating electrode vessel has been applied in order to accelerate the attainment of equilibrium.
Measurements of the potential are made by the use of a suit able type of potentiometer, the readings of which are calibrated by means of a standard cell. If the solution to be measured has a low resistance a pointer type of galvanometer with a sensitivity of 2 micro-amperes for I° may be used, but for general purposes a more sensitive portable mirror galvanometer with combined lamp and scale is more suitable. The hydrogen electrode is not applicable to solutions containing any material which interacts with the hydrogen on the surface of the platinum, or which exert a "poisoning" action on the platinum. The action of the electrode is in this way vitiated by the presence of ammonium salts, nitric acid, nitrous acids and other reducible substances, salts of metals more noble or electropositive than hydrogen, unsaturated organic bodies such as acrylic, crotonic, fumaric and maleic acids, also chloroacetic and similar acids, and sulphur dioxide and carbon dioxide at high concentrations. With these exceptions the hydro gen electrode can be applied to all strong and weak inorganic and organic acids, either in the free state or partly neutralized, and in so-called buffered solutions (where the hydrion concentration is held approximately constant), the e.m.f. is well defined on the alkaline side of neutrality from 7 to 12.
Quinhydrone Electrode.
This system depends on the equilibrium which is attained through the tendency of quinol, formed by the dissociation of quinhydrone, to change into quinone with the liberation of free hydrogen, This hydrogen at an estimated pressure of atm. is liberated on a platinum wire or foil immersed in a solution containing quin hydrone, and develops instantaneously an e.m.f. of a magnitude determined by the hydrion concentration of the solution. On account of this extremely low though constant pressure of hydro gen, the mechanism is not disturbed by the presence of reducible substances which cannot be measured accurately by the usual hydrogen electrode.The quinhydrone electrode has proved to be of particular value for measurements of substances which have a poisoning effect on the hydrogen electrode, while with physiological fluids its meas urements are not disturbed through the displacement of car bon dioxide by hydrogen. For other purposes, such as electro metric titrations, advantages it possesses are due to the equili brium potential being set up more rapidly than with the hy drogen electrode, while no outside supply of hydrogen is required. The system cannot be employed accurately, however, with strong alkaline solutions, or those with certain organic compounds which react with the quinhydrone and modify the potential. In many of these instances, however, advantage may be taken of the slowness of the reaction with the quinhydrone, and reliable determinations may be made shortly after mixing the substances and admitting to the electrode cell. The arrangement of the electrode system is shown in fig. 2.
The liquid to be measured is placed in the tube M. For accurate work with liquids nearly neutral this tube needs to be of special resistance glass. A quantity of o.05-0.05 grams of specially pre pared quinhydrone per io cu.cm. of solution is added and, after inserting a glass stopper, the contents of the tube are shaken until the quinhydrone is dissolved. A platinum wire 41 in. long and 0.3 mm. diameter wound as a spiral and sealed in a glass tube is then inserted, when a potential difference is instantly de veloped at the junction of the metal with the electrolyte and, as with the hydrogen electrode, the potential is proportional to the hydrogen ion concentration of the solution. The amounts of quinhydrone specified will give a saturated solution. Only a small influence on the potential is exerted by the actual concentration of the quinhydrone, however, unless selective adsorption or inter action of one of its components occurs.
Measurement is made by employing as a second electrode, either a standard calomel electrode, or else a second quinhydrone electrode surrounded by a buffered standard electrolyte of known hydrogen ion concentration. The solutions around the two elec trodes are connected by tubes to a vessel B containing concen trated potassium chloride solution, whereby boundary potentials are minimized or eliminated. Diffusion is hindered and other ad vantages gained by having the potassium chloride electrolyte present in the connecting tubes set in an agar gel medium. The potential difference between the electrodes of the cell thus formed is determined by a potentiometer as described for the hydrogen electrode. The potential developed by the quinhydrone electrode with any given electrolyte is, at 18° C, 0.7044 volt more positive than the usual hydrogen electrode. When using a saturated po tassium chloride-calomel standard, the value of the hydrion con centrations is, at 18° C, obtained from the equation: where E is the measured potential.
An alternative standard electrode may be formed from a sec ond quinhydrone solution in presence of the buffer mixture o.oiN HC1+0.09 N KC1. With this standard, the relation between and E, is at i8° C given by the equation The general relation for other temperatures is given by the ex pression : The value of the standard electrode is 2.03 and for values of the solution under measurement higher than this, the potential of the standard will be positive relatively to the measuring elec trode, while for values lower or more acid than this the poten tial will be negative. It follows from the above relation that at 18° C a change in of o•oi causes a displacement of the poten tial of o.58 millivolt. With regard to temperature control, to avoid an error greater than o•oi a temperature constancy of C will suffice if the solution to be measured has a value of 3, whereas with a value of 9, it is necessary for the same accuracy to regulate the temperature to within C.
In measurements by the above procedure in concentrated salt solutions, such for instance as those generally employed in the electro-deposition of metals, deviations of potential from the cal culated value may occur on account of the so-called salt error. These may generally be obviated by adding, together with the quantity of quinhydrone which is slightly in excess of the amount required to saturate the solution, an excess also of either quinone or quinol. With quinone a quantity of o•i g. per io cu.cm. of solu tion is necessary to saturate an aqueous solution, while the solu bility of quinol is about i g. in io cu.cm. With these quinone quinhydrone and quinol-quinhydrone cells, the single electrode potentials are different from those of the quinhydrone electrode, but if the same medium is employed around both electrodes, the combined potential of the cells is the same function of the hydro gen ion concentration as is given in the above equations. In selecting the system to be employed, it may be taken that accurate values are as a rule indicated by constancy of potential over a long period.
Measurements in Acetone-water Solvents.
A good solv ent for a large number of compounds which are insoluble in water is given by a mixture of acetone and water containing io cu.cm. of water in Ioo cu.cm. of the mixture. In this medium a solution containing o•oo5N HC1+o•oo5N KC1 is found to have a of 2.7 at 18° C. If a quinhydrone electrode composed of this electrolyte is employed as a standard, the value of the solution under measurement is given by the equation PH = E 0.0577 The apparatus is connected as illustrated in the figure. For the purpose of accurate measurements the standard electrode ves sel S is provided with three electrodes each containing platinum foil a in. Xi in. X 0.05 mm. thick, curled round to about 4 in. diameter. As a precautionary measure readings may, in this way, be taken with each of the multiple electrodes in turn. Only con tamination of the platinum leading to error, such as by traces of mercury from the tube above, is thus detected by its disagree ment with the remaining electrodes. A 3.5N solution of potassium chloride is employed for filling the intermediate connecting bath B.
Glass Electrodes.
The use of glass electrodes for the deter mination of hydrogen-ion concentrations has been developed by P. M. T. Kerridge (J. Sci. Instr., 1926, p. 404). This method depends on the principle that, if two solutions of different hydro gen-ion concentrations are separated by a thin glass membrane, a difference of electrical potential will be found between the two sides of the glass which is proportional to the ratio of the loga rithms of the hydrogen-ion concentrations on the two sides, ac cording to the Nernst formula. The glass behaves as a solid elec trolyte though of a very high resistance, but the mechanism of the reactions is not fully understood. Many types of glass are suitable for this work, excepting those rich in borosilicate. A German soft soda glass has been most frequently used. The membrane should be about 0.025 to 0.030 mm. in its thinnest part and is preferably formed as a bulb inside another larger and thicker bulb. Contact with the solutions in the glass electrode is made by means of two calomel electrodes opposed to one another.The general arrangement of a convenient type of apparatus is shown in fig. 3. An earthed metal stand is fitted with a rack and two pinions so that the levels of the electrodes may be easily altered at will. The calomel electrodes are held in sleeves ground to fit, to which are attached short lengths of glass rod fixed into insulating blocks of amberite or orca, these being similarly fixed to the stand. Diffusion of potassium chloride into the solutions in the glass electrode is prevented by small ground caps fitted over the tips of the calomel electrodes, and the two taps un greased in the middle race are turned off while the measurements are being made. The resistance thus introduced is not great corn pared with that of the glass membrane. The glass electrode is placed on a glass plate carefully insulated by amberite or orca from the base of the stand.
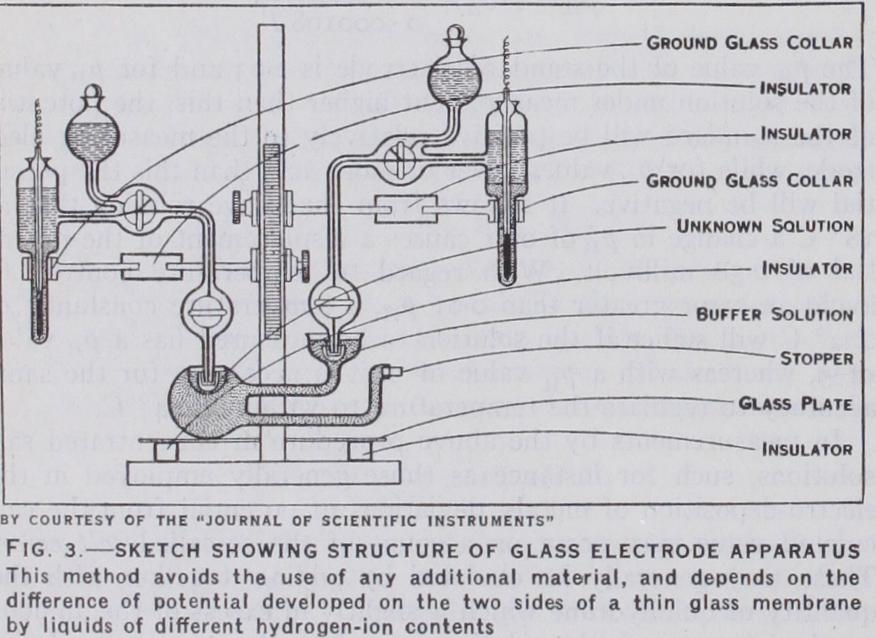
As the resistance of the glass membrane is very high, it is neces sary to use an electrometer as null point indicator in the poten tiometer circuit. The most suitable potentiometer is that of Lindemann. It is necessary to take precautions to shield the ap paratus from electrostatic charges and to ensure that the insula tion resistance in the part of the circuit between the glass elec trode and the electrometer is especially great. The buffer solution to be employed on the inside of the membrane vessel may con veniently be a mixture of potassium phosphates of about 7. The electrode may be standardized by replacing the unknown solu tion by a N/2o solution of potassium hydrogen phthalate, and measuring the potential obtained. The of this solution is 3.97 at 18° C. If E, is the potential found with potassium hy drogen phthalate in the electrode, and E.,• with the unknown solu tion, then the p$ value of the unknown solution PHx is given by the relation This method has been applied largely in physiological research for determinations of hydrogen ions in fluids with which accurate measurements cannot be made by other types of electrodes.
