Invertebrate Embryology
INVERTEBRATE EMBRYOLOGY. The word em bryology means literally the science of the fruit of the womb (Greek but in practice its meaning is extended to embrace all forms of individual development or life-history, whether that development takes place within the mother's womb, as in a human being, within a shell, as with a chicken, or freely in the open, like the frog tadpole.
The term invertebrate is a comprehensive one which includes all animals that are not vertebrates. There are two grades of ani mals, viz., the Protozoa and the Metazoa. The first consist either of single cells, or of small masses of protoplasm with numerous nuclei, but not divided into cells, or, finally, of small groups of similar cells connected with one another, all of which are similar to one another and which carry out the same functions in the animal. When the animal reproduces they all take part in this act, so that the mother disappears in giving rise to her offspring. The bodies of the Metazoa, on the other hand, are composed of cells which are not all alike, but which are differentiated into sheets called tissues, which have different tasks to fulfil in the economy of the animal. When reproduction takes place this is carried out only by some of the cells, and so a distinction is made between the "germ-cells," which undergo development and give rise to new individuals, and the "soma" or body which carries out the other functions, such as digestion, movement, excretion, etc., and which shelters and nourishes the germ.
The life-history of the Protozoa (q.v.) exhibits essentially the same phenomena as that of the Metazoa, but for practical considerations it is not included in an account of the embryology of the Invertebrata. The Metazoa include about 16 distinct groups of animals, termed "phyla" (Greek Of)Xoy, race). One of these phyla consists of the Vertebrata or back-boned animals to which we ourselves belong; all the rest are called Invertebrata.
The invertebrate phyla are (I) the sponges (Porifera, q.v.), (2) the sea-anemones, corals, polyps and jelly-fish (Coelenterata, q.v.), (3) the flat-worms (Plalyhelminthes, q.v.), (4) the pro boscis-worms (Nemertea), (5) the thread-worms (Nematoda, q.v.), (6) the arrow-worms (Chaetognatha, q.v.), (7) the seg mented worms (Annelida, q.v.), (8) the articulates or jointed legs (Arthropoda, q.v.), (9) the shell-fish and cuttle-fish (Mol lusca, q.v.), (io) the spiny-skinned radiates (Echinodermata, q.v.), (I I) the wheel-animalcules (Rotif era, q.v.), (12) the moss animals (Polyzoa, q.v.), (i3) the foot-axis animals (Podaxonia), (14) the lamp-shells (Brachiopoda, q.v.) and (15) the Entero pneusta (q.v.), worms, breathing by slits in the wall of the gut, which constitute a bridge between the Invertebrata and the Vertebrata.
The reproduction of the invertebrate Metazoa takes place by two methods,—by budding or fission and by sexual germ-cells or gametes. Owing to considerations of space we can only consider reproduction by germ-cells.
The gametes are of two kinds, termed male and female, which normally must unite with one another before development is possible. The male cells are produced by a special type of indi vidual called a male, the female cells by another type, the female. Occasionally both are formed in the same individual, which is then termed a hermaphrodite. The product of the union of a male and a female gamete is a zygote. The general structure of these cells is remarkably constant throughout the whole range of the Metazoa, from the sponges up to man himself. The female cell, the egg or ovum, has stored in its protoplasm deposits of reserve material or yolk, which enable the zygote to carry on its existence till it is able to obtain food from the outside.
These deposits vary enormously in bulk and bear little or no relation to the size of the future organism, but solely to the time which must elapse before food is available from outside, and the amount of the deposit regulates the size of the egg. Thus the egg of a woman is only L in. in diameter, whereas that of a hen (termed "the yolk") is, as we all know, the size of a golf ball. When an egg has very little yolk it is termed alecithal. When there is a good deal of yolk massed towards one pole the egg is said to be telolecithal; when the yolk is in the centre sur rounded by a skin of protoplasm, the egg is called centrolecithal. The Echinodermata (star-fish, sea-urchins, etc.) have, with few exceptions, alecithal eggs; the eggs of Mollusca (snails, clams and cuttle-fish) are telolecithal, whilst those of Arthropoda (crabs, spiders, insects, etc.) are centrolecithal.
The male gamete, the sperm or spermatozoon, is, when young, a simple rounded cell indistinguishable from a young female cell, but as it grows and ripens it undergoes remarkable changes. A vibratile filament termed the tail grows out from close beside the nucleus; the nucleus shrinks, expelling the nuclear sap and be comes converted into a small dense mass, termed the head, and the whole of the protoplasm is then sloughed off. The sperm, in virtue of the possession of a tail, is endowed with the power of locomotion; its task is to seek the female cell and to unite with it. When the head touches the surface of an egg the screw-like motion of the tail drives it in. As soon, however, as it is immersed in the protoplasm of the egg, the egg reacts by producing a mem brane at its surface termed the vitelline membrane, which may be regarded as the primitive egg-shell, within which the zygote completes the first stages of its development. This membrane, in most cases, cuts off the tail of the spermatozoon, and in all cases prevents more spermatozoa from entering, so that all the potencies of the father who produces the male germ-cell are con veyed into the egg by a single nucleus, a fact which has a most important bearing on theories of heredity (q.v.). Once inside the egg, the spermatozoon head absorbs fluid and swells up to form a normal nucleus. (See CYTOLOGY.) The two nuclei now approach one another and become placed side by side to form the compound nucleus of the fertilized egg or zygote. In some cases the two components, male and female, can be distinguished from one another in the zygote nucleus during the first divisions of the egg, and it is then seen that the plane of division always cuts male and female portions of the nucleus symmetrically, so that equal portions of paternal and maternal nuclear substance are distributed to each daughter-cell. (See REPRODUCTION.) Both the eggs and the male cells in all Metazoa divide twice during the process of ripening; these divisions in the male-cell give rise to four equal spermatids, all of which develop into sperma tozoa, but in the case of the female germ cell they give rise to the ripe egg and to three minute vestigial cells termed polar bodies. The polar bodies, or at least one of them, adheres to the egg for some time, and the area to which they cling is known as the animal pole of the egg; the opposite pole towards which yolk is massed is known as the vegetative pole. In small and moderate sized eggs the spermatozoon enters at the vegetative pole and makes its way upwards towards the female nucleus, which de cends to meet it after having divided to form the second polar body ; the path taken by the entering sperm seems to fix the future median plane of symmetry of the embryo.
There are two phases of development, such as are exem plified by the unhatched chick and the frog tadpole : they are termed respectively the embryonic and the larval phases. In the embryonic phase, the young organism is protected from the out side world by an egg-shell or within the womb of the mother and derives its nourishment either from stores of yolk or from the mother's womb. In the larval phase on the other hand, the young animal moves freely about and has to escape from its enemies and obtain its food by its own efforts. In every life-history there is both an embryonic and a larval phase; for no egg is ever ,hrust out naked into the world. Every one during the earliest part of development, as we have already seen, is protected by a vitelline membrane; and no animal is hatched exactly like the adult parent. Every animal undergoes a shorter or longer period of growth after birth during which it ,changes its shape and pro portions and this part of its life-history may justly be called a larval phase. The first question to be settled is how these two phases are related to one another—which is the primary and which is the derived one? A comparison with one another of allied species leaves no doubt that the larval phase is generally the primary mode of development and that the embryo is usually merely a concealed and modified larva. This could be proved by instances selected from any invertebrate phylum, but more familiar cases are seen amongst the vertebrates. Everyone is acquainted with the tad pole larva of frogs and toads, and with its fish-like organs adapted to its life in water. The tadpole has three feather-like gills pro jecting from the head on each side, and between these gill-clefts, reaching into the throat, it has a flattened tail fringed with a fin. Now, in the West Indies there is a Hylodes, which lays its eggs far from water. From these eggs there emerge, not tadpoles, but froglings ready to take up their life on land. Nevertheless, if the covering of the egg be dissected off half way through development there will be found inside it a tad pole with gills and tail. These organs have no meaning in the embryonic life—they can be explained only if we assume that the embryo was formerly a larva.
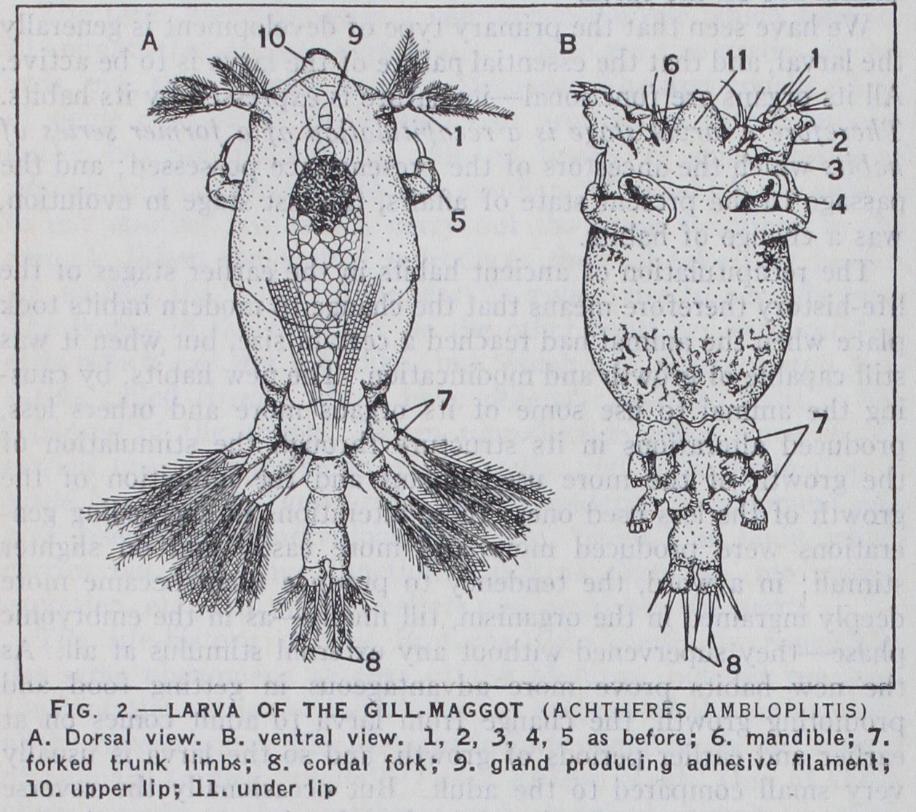
We are now faced with the question, what is the meaning of the larval phase? If we base our reasoning on a broad compara tive view, we shall be driven to the conclusion that the larva is a recapitulation of a former adult condition of the race. We find amongst the invertebrate phyla a typical anatomical structure common to the overwhelming majority of the members of each group, but there is always a minority of species with aberrant structure. When we examine the young stages of these aberrant members, i.e., their latest larval phases, we find that during this period of their life they possess the typical structure of the group. As no comparative anatomist doubts for a moment that the aberrant members have been de rived from more typical ancestors, this means that these aberrant forms in their later development re peat or recapitulate the history of the race. A good example of this phenomenon can be found amongst the oar-footed Crustacea (Copepoda). Certain species such as Achtheres attach them selves to the gills of fish and nourish themselves by sucking the blood. These develop into shapeless maggots devoid of limbs—ex cept the third pair of jaws with which the copepod clasps the gill filament of the host. But when first hatched from the egg, Ach theres is a free-swimming copepod with a typical head, thorax and abdomen and two pairs of swimming legs. In such cases, where the immediate ancestry of the species is clear and undis puted, the meaning of the larval phase is also quite clear, and we are therefore justified in adopting the same explanation for earlier larval stages, corresponding to which living adult forms embodied in other species are no longer to be found. Behind the first larval stage we come to the embryonic stages, to which a similar explana tion must be applied, and so we are led back step by step to the fertilized unsegmented ovum, which on this interpretation repre sents the original unicellular protozoon from which all the higher animals are descended.
But if the whole course of development is thus to be regarded as a series of superposed recapitulations, these are by no means close and accurate reproductions of the ancestral history which they represent. Like human memories, they have suffered blur ring and distortion, and like human memories also, the earliest ones have suffered most, and we shall presently study the principal influences which have brought about this distortion. But since the life-history of every species in the same phylum is a separate edition of the same history, and since the distorting influences vary from species to species, by the aid of a truly comparative embryology we can eliminate most of these secondary alterations from the primitive history and thus restore—not in minute detail, but in broad outline—the general course of the development of animal life on the earth.
We have seen that the primary type of development is generally the larval, and that the essential nature of the larva is to be active. All its organs are functional—its nature is expressed by its habits. Therefore a larval stage is a recapitulation of a former series of habits which the ancestors of the present race possessed; and the passage to the present state of affairs, the last stage in evolution, was a change of habits.
The recapitulation of ancient habits in the earlier stages of the life-history therefore means that the change to modern habits took place when the animal had reached a certain size, but when it was still capable of growth and modification. The new habits, by caus ing the animal to use some of its organs more and others less, produced alterations in its structure through the stimulation of the growth of the more used organs and the inhibition of the growth of the less used ones; these alterations in succeeding gen erations were produced more and more easily and on slighter stimuli ; in a word, the tendency to produce them became more deeply ingrained in the organism, till finally—as in the embryonic phase—they supervened without any external stimulus at all. As the new habits prove more advantageous in getting food and promoting growth, the change from larva to adult comes on at earlier and earlier periods of growth, and so the larva is usually very small compared to the adult. But occasionally the reverse change has taken place—the adult phase has become more danger ous, and serves not for feeding and growth, but chiefly for mating. In this case the larval stage may be more and more prolonged and the full-grown larva may be actually larger than the adult ; this is the case with most moths and butterflies. In the case of certain salamanders such as the axolotl, the same change has taken place, and here the adult phase under ordinary circumstances is sup pressed altogether; the animal mates and lays its eggs whilst still a larva. By exposing such animals to abnormal conditions, how ever, the lost adult phase may be brought to light. (See META MORPHOSIS : Experimental.) The larva is very small compared to the ancestral stage which it represents. For instance, organs repeated in series which act together and form a physiological unit, are represented in the larva by shortened series—sometimes organs which are in pairs are represented only by one. Thus the larva of Achtheres has two pairs of swimming limbs, whereas the normal copepod has four pairs. Occasionally the normal type of environment to which the larval organs are adapted has been changed. This difficulty may be met in two ways ; either the larval phase is transformed into an embryonic one—as in Hylodes—or the larva is forced to change its habits, and then its ancestral features may be almost entirely obscured. This is the case with many larvae of insects.
In the actual history of the race, the transition from one set of habits to another and the accompanying changes of structure must have been gradual ; but it often happens that in the course of time the transitional habits are not suited to the available environment and the corresponding stage is very rapidly passed through. Dur ing this period of rapid change the animal takes no food, and relies on the stores accumulated in the previous stage. See META MORPHOSIS.
In embryonic stages yolk impedes the process of cell-division. In the normal cell this process is accompanied by a stiffening of the protoplasm ; the two daughter-cells at the moment of division seem, as it were, to round themselves off by a kind of coagulation. This stiffening does not last, however, and after the division is completed the protoplasm again becomes semi-fluid and the two cells flatten themselves out against each other. When much yolk is present it acts exactly like water mixed with honey ; it dilutes the protoplasm. Hence the amount of stiffening previous to cell division is much less, the division proceeds slowly and the cells produced are clumsy and large. During the period of relaxation, the daughter-cells often fuse with one another and the process of division is undone. When very much yolk is present, cell division becomes quite impossible and only division of nuclei takes place.
In such eggs, where the protoplasm is concentrated at one pole, development proceeds rapidly in this area, whereas at the opposite pole it is at first at a complete standstill. So the embryo gradually acquires the features of the adult, whilst bearing attached to it a huge sac filled with yolk. Such eggs are termed meroblastic, as opposed to holoblastic eggs, in which the whole egg is divided into cells. The yolk-sac is eventually surrounded by a layer of cells budded from the edges of the developing area ; and as the organs of the embryo become formed the yolk is gradually digested, and conveyed by blood-vessels to the growing part of the body. The yolk-sac shrinks and its covering is eventually incorporated in the skin of the young animal. Amongst invertebrates meroblastic eggs are found amongst Cephalopoda (cuttle-fish), and it is a ludicrous sight to see a miniature squid attached to one pole of a large egg.
The organs of the embryo, as opposed to those of the larva, are functionless, and where they are not transformed into adult organs they are apt to be almost entirely suppressed ; thus in the embryo of the earthworm no trace remains of the broad equatorial belt of cilia, which is so characteristic of the trochophore larvae of other worms and which forms their principal means of locomotion. So, too, in the embryonic development of most snakes no traces of the ancestral limbs are preserved.
The embryonic phase can be divided into three stages, which are respectively (a) segmentation of the egg, (b) formation of germ layers, and (c) organogeny.
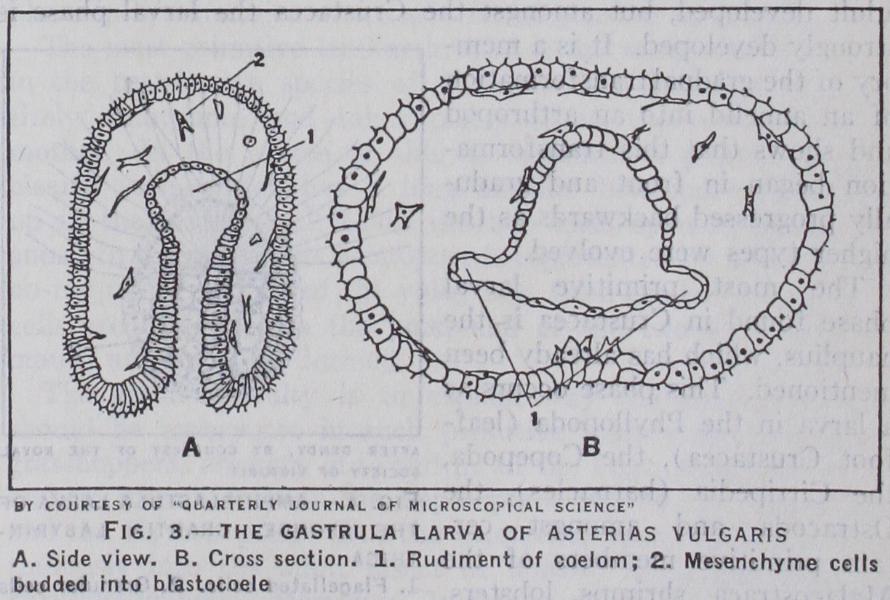
The segmentation of the egg means its division into a large number of cells or blastomeres. In the eggs of the most primitive creatures, in which there is little or no yolk, the blastomeres are almost equal in size to one another, and as they increase in number they separate from one another in the centre, so that they become arranged like a balloon round a cavity. This cavity is called the blastocoele or primary body-cavity, and the whole balloon is known as the blastula. In the case of the Echinodermata, Driesch has proved that the cells of the blastula not only look like each other, but are really like each other in their potencies, for if the blastula be cut into pieces any piece of sufficient size will round itself off so as to form a new blastula of reduced dimensions, which will develop into a perfectly normal larva.
When the distorting influence of yolk is absent or not much developed, the blastula stage can be detected in life-histories be longing to all the invertebrate phyla. Thus in Mollusca it is clear in the development of the limpet (Patella) and the pond-snail (Paludina), amongst Arthropoda in the development of the fairy shrimps (Branchipus), and of the water-fleas (Copepoda), amongst sponges it is seen in Oscarella, whilst it is almost universal amongst Coelenterata, Echinodermata, Nemertea (proboscis worms) and Brachiopoda. In the sponge Oscarella, the coelenterate Eudendrium and in Echinodermata, the blastula is a larval stage, and therefore it may be legitimately considered as a recapit ulation of a common ancestor of all the Metazoa. There exists even at the present time a hollow bell-like organism consisting of ciliated cells tied together by protoplasmic filaments. This creature is termed Volvox, and it is sometimes regarded as a compound Protozoon allied to the simple Flagellata.
The "formation of layers" implies the transformation of this balloon into a double-layered cup termed the gastrula, by the in pushing of one of its sides. The inner layer, which is called endoderm, gives rise to the digestive layer lining the gut—the outer layer or ectoderm to the skin. From the inner layer pockets or cellular outgrowths are given off which give rise to body-cavity and muscles and these are termed the mesoderm. Ectoderm, endo derm and mesoderm are the three germinal layers. The gastrula can be recognized as a stage in the development of all Metazoa except sponges. It exists as a larva amongst the Echinodermata and, in modified form, amongst Coelenterata, in which it is known as the planula. The inner layer in the most primitive forms arises as a process of in-bending or invagination. It looks exactly as if it were pushed in by an invisible finger. The opening produced by this process is called the blastopore. The process of invagination can be analysed into three factors. There is (a) a process of growth and multiplication of cells at one point on the blastular wall which produces lateral pressure; (b) an alteration in character of the cells of the blastular wall. Although after the process of invagination has begun the cells at the animal pole look just the same as before, they have lost some of their original powers—for Driesch has proved that if the upper part of the blastular wall be cut off after this event, though it is able to round itself and form a new blastula, this blastula is incapable of further development; it has become ectoderm. The new cells formed in the zone of growth are from the beginning divided into two categories, one being ectoderm and the other endoderm; (c) a tendency to flow inwards towards the interior of the blastocoele. It is obvious that mere lateral pressure due to increased growth would be relieved by a bending outwards as well as by a bending inwards, and Driesch has shown that under abnormal circumstances this can actually happen with the gastrula of the sea-urchin. There must, therefore, be a special factor in normal development to ensure that invagnation or bending inwards takes place. This we may call inward cytotaxis.
The planula differs from the typical gastrula in that the in growth of endoderm is a solid mass of cells, not a finger-shaped tube. This mass subsequently becomes hollowed out by the absorption of its central cells and forms the lining of the gut. Indeed, the Coelenterata might be regarded as retaining through out their lives the gastrula type of structure. In some Coelen terata, such as Aurelia (the common jelly-fish), and Urticina (a common sea-anemone), the planula is replaced by a hollow gas trula and the blastopore becomes the mouth of the animal. If we have to decide whether the planula or the hollow gastrula gives the most faithful recollection of a distant ancestor, we must decide in favour of the gastrula. For in the history of the race there must have been continuity of function; at every stage in the process the animal must have been a working machine. Now, a solid mass of cells could not serve as an organ for digesting food, but a hollow tube is adapted to this end. Hence we conclude that the gastrula and not the planula gives us the truest picture of the ancestor.
The sponge Oscarella, as we have already seen, is hatched as a typical ciliated blastula. But it does not become converted into a gastrula. The hinder cells lose their cilia and become granular; in this stage the larva is termed an amphiblastula and the majority of sponges are hatched in this stage. The amphiblastula, like the planula, attaches itself to the bottom by its anterior end, but this end now flattens out and then becomes bent inwards and upwards whilst it is overgrown by a great extension of the granular cells which form the outer dermal layer of the sponge. If we call the inbending of the ciliated cells invagination, then this invagination takes place at the opposite pole from that at which it occurs in the planula. From these ciliated cells the gastral or inner layer of the sponge and the so-called flagellate chambers are derived.
How shall we interpret this metamorphosis? If a blastula-like animal was the ancestor of all the Metazoa, there must have been a remote period in the world's history when the seas swarmed with this type and when nothing higher was in existence. If some of these blastulae, instead of seeking a living in midwater, like the rest of their brethren, descended to the bottom to feed and found it profitable to do so, then in this divergence of habit amongst members of the same strain we should have the explanation of the primary cleavage between the phyla Porifera and Coelenterata. Similar divergences of habit have taken place at every stage in the history of life.
The free-swimming blastulae in their search for food perhaps gradually substituted for a random rolling motion a screw-like motion in one direction, and so the posterior end became special ized for the capture and swallowing of food, whilst the front part became the organ of locomotion. So the gastrula grew out of the active blastula, and the cells at its anterior end being the first to encounter changes in the environment, became transformed into a sense-organ with stiff sense-hairs and alternately gave rise to the first nervous centre. Such a sense-organ has been demon strated by Duerden in the planulae of certain corals. But the very same difference of habit which manifested itself at the blas tula stage, supervened again. Some of the gastrulae sought the bottom and attached themselves there; these gave rise to the great army of plant-like polyps and sea-anemones which consti tute the major portion of the Coelenterata. But there was a minority amongst the Coelenterata which never abandoned the original free-swimming life; these are represented in our present seas by the beautiful comb-bearers (Ctenophora) which propel themselves by eight radiating bands of cilia arranged in little trans verse combs. These bands culminate in a beautiful sense-organ resting on a nervous cushion—situated, unlike the nervous centres of other Coelenterata, in front. Out of animals somewhat like these the evolution of most of the higher invertebrates has proceeded. The ctenophore is nothing but an active, sensitive, glorified gas trula, and in the life-history of the flat-worms (Platyhelminthes) the memory of this ancestor is preserved as Miller's larva. This larva, after a brief free-swimming life, sinks to the bottom and flattens out and becomes converted into a leaf-like flat-worm or turbellarian crawling on its cilia. Miller's larva is an oval organism with a sense-organ at one pole and along its sides eight ciliated lobes which correspond to the eight meridians of combs in the ctenophore.
In the life-history of the proboscis-worms (Nemertea) a larva termed the Pilidium occurs, which also seems to be a memory of a ctenophore-like larva. It is shaped like a helmet with a strongly developed sense-organ at its apex. Below there is a wide sac-like gut opening by a wide blastopore. The ciliated ribs have coalesced into a wavy circular band which, however, retains two side lappets like the lobes of Miller's larva. The pilidium, however, is transformed into the Nemertine worm by an abrupt metamor phosis which, doubtless, represents an extreme compression of an originally long life-history, and which it has been impossible up to the present to interpret satisfactorily.
The trochophore larva which appears in the life-history of not only the shell-fish (Mollusca) but also the Polyzoa and the seg mented worms (Annelida) is a further development of the cteno phore-like gastrula. The typical trochophore is an almost spheri cal larva, with an apical sense-organ at one pole essentially similar to the sense-organ of Miller's larva and the Pilidium. Round the equator is a belt of powerful cilia, by the aid of which the larva moves. This band is termed the prototroch: Wilson has shown in the case of Patella and Waltereck has proved in the case of the worm Polygordius that this ring consists at first of four separate lozenge-shaped patches, in which the cilia are ar ranged in transverse combs. The trochophore may thus represent a four-ribbed ctenophore-like ancestor. Below the prototroch there is, as in the Pilidiudn, a sac-like gut opening by a wide blastopore, which changes into a narrow longitudinal slit, that be comes closed up so as to form a seam-like ciliated groove, except in front, where an opening persists which forms the mouth. In the primitive worm Polygordius there is an opening also at the hinder end of the seam which is the anus. In the case of other trochophores, the anus is usually formed as a new opening at the hinder end of the seam. In these processes we clearly have blurred memories of a history which converted the wide single opening into the gut possessed by the Coelenterata and the flat-worms (Platyhelminthes), into two openings, a mouth for taking in food and an anus for ejecting waste. Since the nemertine worm also has an anus, the pilidium larva represents only the early stage of the trochophore ancestor before the division of the blastopore and the later stage is obscured by the metamorphosis.
The second great advance shown by the trochophore is the formation of a true mesoderm. Two large cells, which in the early trochophore form part of the wall of the hinder part of the gut, are ejected into the primary body-cavity or blastocoele. Here each cell begins a process of active growth and multiplica tion. The daughter-cells are budded off in front, whilst the original mother-cell remains behind; in this way a band-like mass of cells, the so-called mesodermal band is produced, which devel ops a cavity termed the secondary body-cavity or coelom. From the wall of this cavity the germ-cells are developed, and it devel ops tube-like extensions which reach the exterior and form the so-called coelonsiducts. Some of these serve as genital ducts (their primary function) and others secondarily acquire an excretory function. In front of the coelom a pair of string-like ingrowths of the skin or ectoderm cells become hollowed out to form vesicles or tubes. These are the primary excretory organs or nephridia.
The coelom turns up in other phyla where the trochophore larva is not formed, as for instance in the arrow-worms (Chaetog natha) and in the Brachiopoda. In these two phyla it is formed as a pair of hollow pouches of the gut, and on the same principles of interpretation which we employed in the case of the planula, we regard the out-pouching of the gut as the primitive method of forming the coelom, and the ejection of cells from the gut wall as a secondary modification of this process.
If we now follow the trochophore in its gradual process of development into a worm and a mollusc respectively, we find that the change in the first case consists in the gradual lengthening of the body behind the prototroch. The intestine becomes drawn out into a long tube and the mesoderm into a long band. This band becomes broken by cross fission into a series of blocks, in each of which a section of the coelom is formed by the absorp tion of the central cells. The skin becomes indented by a series of grooves corresponding to these blocks, and so the well known appearance of the segmented worm is produced. The prototrochal cells are then absorbed or cast off and the worm sinks to the bottom and begins its burrowing life, varied by brief seasons of spasmodic activity, during which it swims through the water by undulatory movements. These movements are carried out by alternate contractions of the longitudinal muscles on both sides, and these muscles are developed out of the lateral walls of the segments of the mesodermal bands. When, however, the tro chophore develops into a mollusc a different set of changes super vene. The body behind the prototroch becomes arched dorsally into a protuberance known as the visceral hump, inside which is contained the larval stomach. On the apex of the hump a saddle-shaped groove of thickened cells makes its appearance. This is the so-called shell gland, and by this gland a horny cap, the first rudiment of the shell, is secreted. On the ventral sur face of the post-trochophoral body, a ciliated wedge-shaped pro jection grows out, along the line of the ciliated groove which marks the position of the sealed-up blastopore. This is the foot, by which the mollusc moves. In primitive Mollusca this move ment is just a gliding by means of cilia over the bottom, but in more advanced and larger forms the ciliary movements are replaced by muscular wrigglings. We can now catch a glimpse of the divergences of habits which led to the separation of the two great groups of Annelida and Mollusca. The trochophore stock, like the more primitive stock represented by Miiller's larva, sought their food on the bottom. Under these circumstances two dif ferent modes of life were open to them. They might either seek their food in the most superficial layer of the bottom deposit, and in this case they became molluscs; or they might burrow into it, and in this case they became segmented worms (Annelida). But, as we have already pointed out, the Annelida really lead a double life ; in all the more typical forms burrowing alternates with rapid swimming excursions through the open water. The accen tuation of this later habit, and the gradual forgetting of the tendency to burrow led to the evolution of the great phylum Arthropoda, or jointed-leg animals, which contains about three quarters of the known species of living animals.
The Arthropoda differ from the Annelida in the much greater thickness of the cuticle. The coelom has disappeared, leaving only occasional pocket-like remnants, so that the large body cavity of the arthropod is really a blastocoele. In the early embryonic development of all the Arthropoda there are de veloped two well-marked mesodermic bands, just like those of Annelida, and in these transverse segmentation and coelomic pockets appear; in a word it might be said that the embryonic arthropod is an annelid. The three great groups of living Arthro poda, the Crustacea, the Arachnida and the Insecta, differ amongst themselves very much as to the extent to which the larval phase is developed. The Arachnida, except in their most primitive forms, leave the egg-shell with practically all the organs of the adult developed, but amongst the Crustacea the larval phase is strongly developed. It is a mem- .
ory of the gradual transformation of an annelid into an arthropod and shows that this transforma tion began in front and gradu ally progressed backwards as the higher types were evolved.
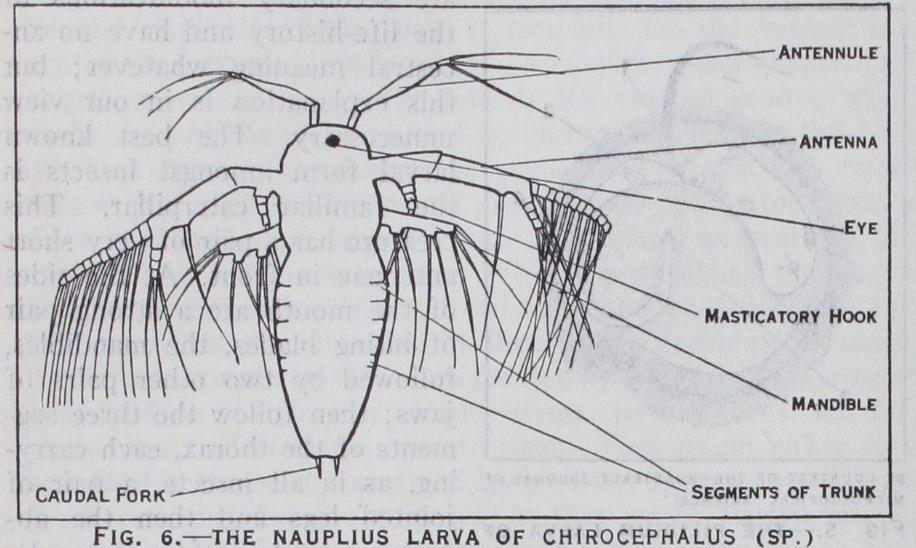
The most primitive larval phase found in Crustacea is the nauplius, which has already been mentioned. This phase occurs as a larva in the Phyllopoda (leaf foot Crustacea), the Copepoda, the Cirripedia (barnacles), the Ostracoda and amongst cer tain primitive members of the Malacostraca, shrimps, lobsters, crabs, etc. As an embryonic stage it can be detected in the life-history of the highest Crustacea. The nauplius is an oval or cylindrical larva with a single simple eye in front and three pairs of jointed legs, one pair slightly in front of the mouth and the two pairs behind it. The mouth is a transverse slit on the under side overhung by a large lip or labrum. The first pair of limbs consists of a straight rod on each side carrying sensory hairs and is termed the antennule: the second and third pair are forked, each limb consisting of a basal piece from which inner and outer branches spring, each having two or three joints. The basal piece on each limb carries an inwardly directed hook which the larva uses for seizing its food. These hooks are termed masticatory hooks. The second pair of limbs eventually becomes the antennae of the adult, whilst the last pair become changed into the mandibles. In most nauplii the body behind the mouth is totally unsegmented but, as already noted, in the nauplii of the primitive Phyllopoda this region is long and cylindrical and marked out into a multitude of very fine ring-like segments. We may interpret this larva as a reminiscence of an ancestral worm, in which the first two or three segments had developed appendages suitable for grasping food, and in which the cuticle of this region was greatly strengthened, but in which the hinder part of the body retained largely its soft, worm-like character. It must be remembered that the marine worms have soft wing-like out growths from their segments known as parapodia, and it is these that are converted into the limbs of arthropods when the cuticle becomes thicker.
Since an arthropod is confined within a rigid cuticle, growth is only possible when this cuticle is thrown off. So the life-history presents the appearance of a series of stages of fixed appearance, known as instars, separated by shorter stages known as moults, during which changes in appearance take place. Of course these changes are not really as sudden as they appear, for they have been gradually going on in the soft tissues underneath the cuticle, but they only become manifest when the cuticle is shed.
Want of space forbids us to describe in detail the various moults by which the nauplius larva transforms itself into a phyllopod, a copepod, a cirripede and an ostracod respectively. We propose to give only a brief account of the development of the Malacostraca.
In only the most primitive shrimps such as Penaeus is the young organism hatched as a nauplius ; in the vast majority of cases, this stage is passed over within the egg-shell and the animal is born as a larva of quite a different kind, termed a zoaea. In the lobster even the zoaea stage is passed through before hatching; the young lobster begins its career as a larva of a more advanced type known as the mysis larva, but in the case of all the shrimps and crabS, the zoaea is the typical larva.
The zoaea is divided into an anterior rounded portion, the cephalo-thorax, and a hinder segmented portion ending in a broad tail fin, the abdomen. The cephalo-thorax ends in front in a spike or rostrum ; at the sides of this are two large eye-stalks bearing compound eyes, whilst at the base of the rostrum, the single eye of the nauplius can still be detected. The cephalo-thorax is covered by an undivided shield termed the carapace, the sides of which form free flaps hanging down at the side of the body; between these flaps and the body is a groove in which are the first traces of the gills. Of the naupliar appendages, the antennule has shrunk very much in size, the antenna retaining its forked condition only in the primitive zoaea of Penaeus. In the zoaea of the common crab, for instance, as in most zoaea, the outer branch of this limb has assumed the adult form of a scale, the so-called squame. The mandible is reduced to its basal stump. Behind the mouth come two segments carrying the two additional pairs of jaws, the maxillules and the maxillae, which are common to all Crustacea. Behind the second of these is the groove which marks the limits of the head region, as distinct from the thorax. The segments of the thorax are exceedingly compressed—only the first two carry limbs ; these are large forked appendages and are used in swimming. In the zoaea of the shrimp the first three pairs carry limbs. The abdominal segments are without ap pendages.
No adult crustacean exactly like the zoaea is found living. But if we watch how the larva uses its limbs, we see clearly that it represents a stage in functional evolution between the primitive arthropod represented by the nauplius and one of the higher Crustacea. In the primitive arthropod, swimming and getting food were functions carried out by the same limbs, but when the arthropod became a crustacean, three of the pairs of limbs, mandibles, maxillules and maxilla, were changed into jaws and the swimming function was relegated to the two or three pairs of limbs behind them which in later life became the foot jaws or maxillipedes. The fact that in the crab zoaea the hinder seg ments of the thorax and the segments of the abdomen have no limbs may be explained, in the same way as their absence was explained in the nauplius, by the assumption that in the ancestor they were thin and undifferentiated and of little functional im portance. In the primitive zoaea of Penaeus the second antenna remains, as in the nauplius, large and forked and still aids in swimming as it does in many of the lower Crustacea, and only one pair of maxillipedes is developed; this represents an older ancestral stage than the typical zoaea. Weldon has given a vivid picture of the movements of a crab zoaea; the little creature swims on its back, using the rostrum and a spine which sticks up from its back as a keel ; it rows itself by its maxillipedes and uses the abdomen as a rudder. This last observation of Weldon throws a flood of light on the causes which led to the evolution of the abdomen. Strictly speaking, this term is only applicable in the higher Crustacea, in which the primitive uniform series of limbs behind the mouth is interrupted after the eighth limb, and the thoracic limbs or peraeopods are succeeded by the much smaller abdominal limbs or swimmerets. The primary reason for the differentiation of the abdomen and the dwindling of its limbs seems to have been its use as a rudder.
The zoaea of the shrimp gradually changes into the adult form by a series of moults. In these moults, some of the legs belonging to the hinder segments of the thorax make their appearance, and begin to take part in the swimming function. In extreme cases as many as six pairs of legs, all similar to one another, may eventually be engaged in this function. The larva now bears a striking resemblance to one of the Schizopod shrimps such as Mysis, and for this reason it is known as the mysis-larva. The lobster is hatched from the egg in this stage. The mysis-larva evidently represents an ancestor in which the swimming function has been adopted by more legs than was the case with the zoaea ancestor. In the common shrimp the last four pairs of the thoracic limbs, however, do not develop into swimming organs, but remain as rudimentary buds till the final moult gives them their adult form. The mysis-larva is transformed into the adult shrimp by the appearance of the abdominal limbs and the disap pearance of the outer swimming branches of the thoracic limbs. In the adult shrimp the thoracic limbs are used only for grasping and walking ; swimming is performed exclusively by the abdomen, so that the swimming function which in the nauplius ancestor was performed by the most anterior appendages has been gradu ally passed back until it has reached the hinder end of the body.
In
the life-history of the crab, the mysis stage is missed out, and the zoaea moults f our times, whilst still retaining the same general structure and appearance. But at each moult the hinder thoracic appendages become more prominent, although they are never forked and never become functional. The abdominal ap pendages, too, make their appearance as small buds. Then a final moult takes place at which the zoaea completely changes its shape and becomes a larva of an entirely different type known as the megalopa. This larva strongly resembles a small lobster. It has all the last five pairs of appendages of the thorax as un f orked limbs, and the abdominal appendages are fully developed and forked and adapted to swimming. At the next moult the megalopa changes at once into a small crab, but all the swimmer ets disappear. Now the adult crab possesses four pairs of swim merets in the female and in the male two pairs of modified swim merets. The swimmerets in the female are quite normal in struc ture, but they are not used for swimming, but merely for holding the egg; the swimmerets in the male are modified into styles and are used for transferring the male cells into the female opening. These appendages are only developed as the little crab grows larger and approaches sexual maturity. Now, in the history of the race there is no doubt whatever that the swimmerets of the lobster an cestor became the swimmerets of the crab, and the disappearance and subsequent reappearance of these limbs in the life-history is one of the most curious phenomena of development and throws a great light on the nature of heredity. We see quite clearly that what is inherited is not a structure as such, but a potency or memory which can embody itself in a structure when the func tional necessity for it arises.
The development of insects shows the paradox that the most modified insects have a long larval life and that their larvae are worm-like in appearance, whilst the development of the lowest and most primitive insects is almost entirely embryonic, and they are hatched resembling their parents in most points except insects are Many authorities hold, therefore, that the larvae of nsects are secondary modifications of the life-history and have no an cestral meaning whatever ; but this explanation is in our view unnecessary. The best known larval form amongst insects is the familiar caterpillar. This creature has a pair of very short antennae in front. At the sides of the mouth are a strong pair of biting blades, the mandibles, followed by two other pairs of jaws ; then follow the three seg ments of the thorax, each carry ing, as in all insects, a pair of jointed legs and then the ab domen consisting of ten segments, of which segments II., IV., VI. and IX. carry a pair of soft, un jointed legs. Now, if we examine the lowest and least specialized insects such as the little wingless Machilis, which scavenges amongst sea-weed left dry by the receding tide, we find that they also have short antennae, biting mandibles and an abdomen every segment of which carries a pair of unjointed legs ; so that we see that the larva of a butterfly may be taken to represent an ancestor which had attained the level of the adult stage of Machilis. This conclusion is strength ened by two other sets of facts, viz.: (I) A closely similar cater pillar larva occurs as a phase in the life-history of the wood wasps, which are primitive Hymenoptera utterly different in structure from the butterflies ; (2) if we examine the embryos of insects like the cockroaches, which are hatched as so-called "nymphs" resembling the adult in most features but devoid of wings, we find in these embryos an almost complete series of unjointed abdominal legs. The caterpillar therefore need not be a secondary adaptation, because it occurs as a phase in the life history of widely different orders, and it is found as an embryonic phase in life-histories where as a larval phase it is absent.
The most primitive land arthropod is the worm-like Peripatus; in the best-known species of Peripatus the development is en tirely embryonic and takes place within the oviduct of the mother. In the course of this development a gastrula stage is clearly seen, which has a long slit-like blastopore that closes up in the middle and leaves the two ends open as mouth and anus. In the insect this blastopore is represented by a long groove on the under surface of the yolky egg, from the bottom of which cells are budded into the yolk, and at the two ends of which mouth and anus are formed.
The great difficulty is to explain why the caterpillar stage should be embryonic in such primitive insects as cockroaches, grasshoppers, crickets, etc., and in all the bugs, and larval in the highest insects such as the Lepidoptera (butterflies and moths) and the Hymenoptera. But the caterpillar does not develop con tinuously into the adult insect or imago. A resting stage, the chrysalis or pupa, intervenes. In this stage the larva is protected in a case either secreted by itself or composed of materials col lected by it and stuck together by its secretion. It undergoes profound changes, and becomes changed from a worm-like form to that of an insect with rudimentary wings—in fact it becomes quite similar to the young of a cockroach or a grasshopper when this is just hatched. The wings develop in the primitive forms as external protuberances, in the more advanced forms from the bottom of deep pits. We see, in a word, that the chrysalis of the higher insects corresponds to the nymph of the lower insects. It is exactly as if the insect, having begun as a larva, had gone back into an embryonic stage. Only a tentative hypothesis as to the cause of this anomaly can be suggested. We know, however, that insects of the cockroach and grasshopper type flourished exceed ingly during the Carboniferous period and attained a gigantic size. Such large insects were never again produced. In the period which succeeded to the Carboniferous two catastrophes overtook the world. In the north there was widespread aridity and the pro duction of extensive deserts, whilst in the south there was an equally widespread ice-epoch. One or both of these climatic changes, it may be suggested, forced the insects which survived them to modify their larval development profoundly. In the higher insects the middle period of development was converted into the chrysalis stage, whilst in the lower insects the earlier part of development, the caterpillar stage, became embryonic. The caterpillar, like the lowest insects, is a scavenger. It was retained as a larval form amongst the higher insects because the parent has had the intelligence to find a suitable environment for the larva and to lay its egg there.
The great group of the Echinodermata (spiny-skinned radi ates) have typically life-histories with a very long larval phase and their larval forms are very different from any which we have as yet described. Living Echinodermata consist of four classes of free-living forms, the true star-fish (Asteroidea), the brittle stars (Ophiuroidea), the sea-urchins (Echinoidea) and the sea cucumbers (Holothuroidea). Each of these classes has its char acteristic larva, but all these larvae are obviously modifications of one type. If we take the common sea-urchin (Echinus miliaris) as an example of the Echinodermata, we find that the eggs are small and alecithal, i.e., almost devoid of yolk. They segment rapidly into blastomeres of almost equal size and a typical blas tula is formed, the cells of which are provided with flagella. By means of these it rotates within the vitelline membrane, which it very soon bursts; it then escapes and swims freely about as a larva. Within 12 hours cells are budded from one side of the blastulae wall into the blastocoele ; these cells, which are termed mesenchyme, are a sort of primitive connective tissue. They be come massed in two places, right and left, and within these groups little tridents of calcareous matter are secreted which develop later into the larval skeleton. Before this is accomplished, how ever, one side of the blastula becomes flattened and in the centre of this surface an invagination gives rise to a finger-shaped gut or archenteron and the blastula becomes a gastrula. Within 3o hours, from the apex of this a little bilobed vesicle is cut off which promptly divides into right and left vesicles. These vesicles —outgrowths of the gut—are the rudiments of the coelom. After this event the gastrula becomes converted into what is known as the prism larva. Another side of the larva becomes flattened so that the original sphere becomes a wedge and the tip of the gut bends towards this second surface. In the centre of it there ap pears a shallow funnel which is the rudiment of the mouth and oral funnel or stomodaeum. This funnel meets and opens into the blind end of the gut and so a complete alimentary canal is established. The gut has now become divided by constrictions into gullet, stomach and intestine and the blastopore forms the anus. The cilia are now restricted to a ring around the edge of the surface into which the mouth opens-•--and at the lower edges of the surface two blunt lobes or arms grow out. With the appear ance of these lobes or arms the prism larva becomes a pluteus or "easel-like" larva, as it was first termed by its discoverer, Jo hannes Miller. The arms are supported by long spine-like out growths of the calcareous tridents. They occur along the course of the ciliated band and the first pair are known as the post-oral arms. But other branches of the tridents extend forwards along the sides of the gullet and mouth and here impinge on the ciliated band and give rise to a second pair of arms, known as the antero lateral pair. Johannes Muller not having observed his larva in the living active state, placed it wrong way up, with the pointed posterior end above, and compared the four long ciliated arms to the legs of the painter's easel. In order to distinguish this larva from the somewhat similar larva of the brittle-star it is now termed the echinopluteus.
As the echinopluteus grows, it develops four other ciliated arms, two overhanging the mouth termed the pre-oral--the calcareous rods supporting these grow from a median calcareous centre in the dorsal region above the gullet—and two at the sides behind the antero-lateral called the postero-dorsal—each of these is sup ported by a rod growing from a new centre on the side of the larva. The echinopluteus retains the same general appearance as it grows bigger and older, until at the age of about four weeks it suddenly descends to the bottom and changes into a young sea urchin. Four crescent-shaped outgrowths from the ciliated band, two placed dorsally and two ventrally, are developed; these in later larval life become very large and on them the main part of the swimming function devolves; but these crescents, termed "epaulettes," are peculiar to the genus Echinus itself and some allied genera. Want of space compels us to describe in briefest outline the change of the echinopluteus into the sea-urchin. The coelomic sacs formed in the prism larva become divided into anterior and posterior halves on each side. From the anterior section on the left side a small rounded vesicle termed the hydro coele is budded off. Round this bud the whole organization of the future urchin is built up. The hydrocoele becomes bent into a short thick hoop, the ends of which join so that a ring is formed. This becomes the ring canal of the water vascular system. From the ectoderm opposite the hydrocoele a deep pouch is developed which becomes a closed sac. The roof of this sac is called the amnion. Its floor grows out to form the first spines and tube f eet of the future sea-urchin ; into the tube-feet, of course, branches of the hydrocoele project. Through the centre of the floor a new intucking marks the position of the adult mouth, which has no known relation to the larval mouth. The whole com plex of spines and tube-feet is known as the Echinus-rudiment. From the anterior coelomic sac on the right side a small vesicle is budded off the corners of which lie above the gullet almost in the mid-dorsal line. This sac is called the "pericardium." Its floor is bunched up to form a tube and the space—part of the blasto coele—included between the upper wall of the gullet and this floor is called the heart. The tube-like ingrowth pulsates rhythmi cally and causes the blastocoele fluid to circulate.
As the larva grows older the "Echinus-rudiment" increases in size till it occupies the whole side of the larva. Finally, the "amnion" becomes absorbed and the first tube-feet project freely. Then these feet take hold of the substratum and in a marvellously short time—about half an hour—the larval arms are all absorbed, the ciliated band and ciliated epaulettes vanish. The larval buccal funnel loses its connection with the gullet, shallows out and dis appears, and the easel-like larva is reduced to a minute round flattened disc, which then takes up the ordinary creeping life of the sea-urchin.
The larva of the brittle-stars (Ophiuroidea) is also called a Pluteus. In fact it is the original Pluteus which was described by Johannes Milner. It differs, however, in many important re spects from the larva of the sea-urchin and for this reason it is now termed the ophiopluteus. The eggs of brittle-stars agree closely with those of sea-urchins in their segmentation, blastula and gastrula stages, and in the way in which mouth arms and coelom are formed. In the early pluteus stage, however, the first arms to be formed are not the post-oral, but a pair growing out from the sides of the larva. These arms (occasionally present in the echinopluteus) are directed forwards and are the longest arms of the larva. They are followed by antero-lateral, postero-dorsal and post-oral arms, but no pre-oral arms are formed, so that here again we find an eight-armed larva; but the arms are not quite the same as those of the echinopluteus. The arms are stiffened by calcareous rods, but all the rods on the same side of the larva are outgrowths of the same calcareous star. The coelom is formed in the same way as in the echinopluteus; it divides and gives rise to the hydrocoele bud in the same way; but the history of this bud is rather different. It becomes converted into a ring-canal, and this ring-canal gives off five lobes, but these lobes project, not into an amniotic space, but into the buccal funnel of the larva. As the ophiopluteus grows older, the ciliated arms become absorbed ; but the postero-lateral ones persist and even grow longer. The buccal funnel of the larva shallows out and disappears, thus exposing the first formed tube-feet, which take hold of the substratum and there metamorphosis is com pleted and the creeping life of the adult is begun. Then the postero-lateral arms finally disappear. It is to be noted that here the adult mouth is the opening between the buccal funnel of the larva and the gullet and that no new opening is formed.
The larva of the ordinary starfish is called the bipinnaria and seems at first glance very different from both the echinopluteus and the ophiopluteus. Closer examination reveals its essential simi larity. The egg segments in the same way and the blastula and gastrula stages are the same ; the larval mouth, the coelom and arms are formed in the same way. But the next stage is sausage shaped. This shape is due to the existence of a long forehead or pre-oral lobe in front of the mouth. Into this the ante rior division of the coelom extends on both sides and two sacs fuse into a median sac in front of the mouth. When the ciliated band is formed out of the general ciliated covering of the gastrula, it runs along the sides of the larva to the front end ; arrived here it bends backwards along the underside of the pre-oral lobe. This loop of the band is soon constricted off from the rest and forms a special pre-oral ciliated band. Both bands, the pre-oral and the main one, develop outgrowths, but these are not supported by calcareous rods; they resemble the wings (pinnae) of insects, whence the name bipinnaria. The main band of cilia develops a median anterior "pinna." The division of the coelom into ante rior and posterior portions takes place as in other echinoderm larvae; and the hydrocoele bud develops as in the sea-urchin, but there is no amnion formed and the primitive tube-feet from their first appearance project freely to the outside. The adult mouth is formed as a new perforation in the centre of the hy drocoele ring. What distinguishes, however, the bipinnaria from the other larvae of free echinoderms is the mode of its meta morphosis into the adult. After swimming about for two months, during which time the hydrocoele and its appendages grow larger in relation to the rest of the body of the larva, the bipinnaria de velops at its anterior end between the pre-oral and main ciliated bands a group of three short arms ending in adhesive knobs ; by means of these the larva anchors itself to the sub-stratum. It has now become a brachiolaria. An oval auctorial disc, consisting of sticky cells, is developed at the apex of the forehead between the bases of these arms ; by the contraction of the arms the disc is brought into contact with the substratum (often a bit of sea weed) and so a permanent fixation of the larva is effected and the pre-oral lobe is converted into a stalk. After becoming fixed, the ciliated bands and their pinnae disappear, and the stalk short ens till it becomes a small knob. The buccal funnel of the larva shallows out and disappears, the shortened stalk is attached near the adult mouth to the under surface of the future starfish. Fi nally, as the hydrocoele and its tube-feet become more and more developed, the starfish wrenches its vestigial stalk loose from its attachment and walks away.
The auricularia larva of the sea-cucumbers resembles the bi pinriaria in outer appearance. There is a pre-oral lobe, though not as well developed as that of the bipinnaria ; and the ciliated band is prolonged into a pre-oral loop on the underside of this lobe but this is never quite cut off from the main band. The band grows out into lobes or pinnae, which, like those of bipinnaria, are not supported by calcareous rods. There is a blunt anterior median one, and the postero-lateral pinnae are long and curved and have been compared to little ears (auriculae), whence the name of the larva. The development of the coelomic vesicle is different in the auricularia from what it is in other echinoderm larvae; it remains unpaired and divides into anterior and posterior halves; the posterior portion subsequently divides into right and left halves. The anterior portion, which represents the left anterior vesicle of other larvae, gives rise to the hydrocoele ; this grows round the larval gullet, no separate adult mouth being formed. The auricularia swims about, gradually growing bigger for a fort night, and then suddenly changes into another larval form known as the pupa. This is smaller than the auricularia and its tissues are much more dense and opaque. The first-formed tube-feet grow out into the buccal funnel (larval stomodaeum). When the change into the pupa occurs this stomodaeum deepens and its opening becomes narrowed and practically closed; it is then known as the atrium. The atrium shifts from its original ventral position to the left side of the larva and then by the shrinkage of the pre-oral lobe, the opening of the atrium becomes terminal. At the same time the ciliated band of the larva becomes broken into short pieces which re-unite to form circular bands of cilia surrounding the pupa as the hoops surround a barrel.
The first-formed tube-feet project into the enlarged buccal fun nel or atrium just as they do in Ophiuroidea ; but these tube-feet are not terminal appendages of the radial canals, as they are in the other three classes, but basal ones—the so-called buccal tube-feet, which, in the adult sea-cucumber, are arranged around the mouth and are used to collect food. The radial canals extend along the body of the pupa outside the atrium. After swimming for a few days the pupa loses its ciliated bands and drops to the bottom; it develops a skeleton of calcareous plates almost touch ing one another and recalling the shell of a sea-urchin. The atrium opens and the tentacles protrude and begin to collect food and tube-feet develop on the radial canals, first on those that lie on the under side of the sausage-shaped animal and then on those that lie on the upper side.
From the descriptions just given it seems clear that the bi pinnaria, ophiopluteus, echinopluteus and auricularia are modifica tions of one original type of larva. In our view, this larva is a recapitulation of an original free-swimming ancestor of all the echinoderms, which, like most free-swimming animals, was bi laterally symmetrical. Like the trochophore, the echinoderm larva gives evidence of having passed through blastula and gastrula stages in the history of the race, but there the resemblance ends.
The protoplasm of the cells corn posing the trochophore blastula is already specialized, so that when these cells are separated from their fellows, they pursue, so long as they live, the same development as they would have done in the intact organism. The cells of the echinoderm blastula, on the other hand, are unspecialized ; any suffi ciently large portion of the blastula wall will round itself off and form a blastula of re duced size, which will pursue a normal development and give rise to a small normal larva. The echinoderm larva is, therefore, in a much more primitive con dition than the trochophore larva from which Annelida and Mollusca have very possibly been derived. We saw that the ciliated band of the trochophore was built up of four discrete groups of ciliated cells which very probably represent the "ribs" of a ctenophore ancestor. The ciliated band of the echinoderm larva shows no such indications ; it is not transverse but obliquely longitudinal, crossing the dorsal surface on the pre-oral lobe or forehead and the ventral surface in front of the mouth. The coelom in the trochophore is a derivative of the growth of two large cells—originally part of the gut wall. This method of forma tion represents an original gut-pouch, modified in connection with the extreme specialization of the cells of the trochophore ; in the echinoderm larva the origin of the coelom from a gut-pouch is obvious. Another difference concerns the fate of the blastopore. This in the trochophore closes up in a longitudinal seam, one end of which remains open as the mouth, while in some very primitive forms the other end remains open as the anus. In the echinoderms the blastopore is always a round hole which persists as the larval anus, and the mouth is a new opening formed apparently com pletely independently of the blastopore. To sum up, the trocho phore and the echinoderm larva both represent simple free-swim ming ancestral forms which were, however, markedly different from one another; to trace these back to a common root it is necessary to go back almost to the beginnings of the Metazoa.
The most remarkable feature in the life-history of the echino derms is their change from the bilateral symmetry of the larva to the radial symmetry of the adult.
It is obvious that no light is thrown on its meaning by the sudden metamorphoses of sea-urchins, brittle-stars and sea-cucum bers. In the life-history of the common starfish (Asteroidea), however, a fixed stage intervenes between the free-swimming phase and the crawling adult. Some members of other phyla desert the open water to seek food at the bottom—and in many cases— owing probably to the existence of powerful currents, they develop the habit of holding on to one place and seizing the food as it drifts past them. All such animals tend to develop tentacles on all sides, for instance, the Polyzoa (q.v.). If in the fixed echino derm ancestor the hydrocoele had already developed its tentacles; then these must have been developed on both sides, and in asteroid, ophiuroid and echinoid larvae a second hydrocoele bearing tenta cles is occasionally developed on the right side as well as on the left. But some constitutional peculiarity of the stock favoured the left side at the expense of the right, and so this hydrocoele developed into a ring suppressing the corresponding organ of the right side altogether. The formation of this ring and the func tional importance of the organs developed from it is the founda tion of the radial symmetry ; all the other organs of the body are adapted to it. As the ancestral starfish spread into more tran quil waters, they wrenched themselves loose from their stalks and gave rise to the free living Echinodermata. The Ophiuroidea are only more specialized starfish, and the sea-urchins, when just metamorphosed, are little flat disc-like starfish, in which nothing of the globular form of the adult can be seen. They developed, we may suppose, out of starfish which took to climbing in verti cal crevices—situations which mountaineers call chimneys—and in these circumstances the tips of the arms were bent upwards to get a better grip of the cliffs above them and so the globular form was attained. The sea-cucumbers are perhaps the result of a further development of this habit, for from climbing vertical walls to wriggling amongst stones is an easy passage; but the young post-larval sea-cucumber still show traces of the shell of the sea-urchin ancestor from which it was derived.
There still exist at the present day a few stalked echinoderms, represented by the class Crinoidea—the last survivors of an im mense multitude of Palaeozoic forms, so numerous at one time that vast masses of limestone are formed of their accumulated re mains. The stalk springs from the centre of the back, whilst mouth and anus are both on the opposite surface. The eggs of the only crinoid whose development has been worked out are large and full of yolk, and the earlier stages of development are embryonic, and during the short larval phase the young animal takes no food. Nevertheless, this larva shows in broad outline some resemblance to the bipinnaria, although the cilia are arranged in transverse bands as in the pupa of sea-cucumber. It has a long pre-oral lobe containing a single anterior body-cavity; and at the apex of this lobe there is a sucking disc by which it soon fixes itself and the pre-oral lobe is then changed into a stalk. There is a larval buccal funnel or stomodaeum, which, however, never opens into the gut, since the larva takes no food, and into this funnel the first tentacles project just as they do in Ophiuroidea and Holothuroidea. But the changes which supervene after the larva is fixed are quite different in the Crinoidea from what they are in Asteroidea. The buccal funnel of the larva, instead of being pressed closer to the substratum, as it is in the starfish, is rotated backwards till it reaches the posterior pole of the larva, which is, of course, directed upwards towards the open water. Then it opens and the ciliated tentacles spread out and collect the micro scopic plants and animals that swim freely through it. We now see that the wide distinction between the fixed and free echino derms rests ultimately on a different choice of food. When the ancestral echinoderm began to hold on to the ground with its pre-oral lobe and to grope about in the water with its tentacles for food, two courses were open to it—it might either bend down wards to grasp what was drifting beneath it, or reach upwards to attain what was floating above it—the first course led to the de velopment of the free echinoderms, the second to the crinoids.
If we search through the animal kingdom for larvae resem bling those of echinoderms, we find such a larva only in the phy lum Enteropneusta. This is the tornaria larva of the worm-like Balanoglossus which is described in the article BALANOGLOSSUS. But the resemblance is so close that tornaria was actually re garded as an echinoderm larva until its life-history and meta morphosis were known. It has a longitudinal ciliated band with a pre-oral loop which resembles that of the auricularia. It has a long pre-oral lobe containing a single anterior coelom like the bipinnaria. Besides this coelomic sac, a pair of anterior sacs at the sides of the gullet represents the hydrocoele, and its missing fellow on the right side and a pair of posterior sacs represent the posterior sacs of the echinoderm larva. Most striking of all, it has above the gullet a small pericardial sac, the floor of which vibrates and acts as a heart, exactly as it does in the larvae of Asteroidea, Ophiuroidea and Echinoidea. The tornaria has some additional features. There is a posterior transverse ring of cilia behind the longitudinal ciliated band and an apical nervous plate with two eyes at the apex of the pre-oral lobe. Such a plate is not found in the bipinnaria but is found in the echinopluteus. The fate of the tornaria larva is different from that of the echino derm larva because the adult habits are different, but there can be no serious doubt that it represents the same ancestral stock. An ally of Balanoglossus, Cephalodiscus, actually uses its pre oral lobe for temporary fixation and has the middle section of the coelom on each side developed into long ciliated tentacles. As pointed out in the article BALANOGLOSSUS, this animal in its gill sacs, and rudimentary nerve-tube and notochord, shows unmis takable affinities to the vertebrates, so that here the embryology of the Invertebrata merges into that of the Vertebrata.
In this article we have striven to give some idea of what has already been gleaned as to the past history of life by the study of invertebrate embryology. But what has been discovered is only a small fragment of what is still unknown and could be known if more life-histories were studied and compared.