Calcite
CALCITE, a mineral consisting of naturally occurring calcium carbonate (CaCO3), crystallizing in the rhombohedral system. With the exception of quartz, it is the most widely distributed of minerals, while in the beautiful development and extraordinary variety of form of its crystals it is surpassed by none. In the massive condition it occurs as large rock-masses (marble, lime stone, chalk) which are often of organic origin, being formed of the remains of molluscs, corals, crinoids, etc., the hard parts of which consist largely of calcite.
The name calcite (Lat. calx, calcis, meaning burnt lime) is of comparatively recent origin, and was first applied, in 1836, to the "barleycorn" pseudomorphs of calcium carbonate after celestine from Sangerhausen in Thuringia; it was not until about that the name was used in its present sense. The mineral had, however, long been known under the names calcareous spar and calc-spar, and the beautifully transparent variety called Iceland spar had been much studied. The strong double refraction and perfect cleavages of Iceland-spar were described in detail by Erasmus Bartholinus in 1669 in his book Experimenta Crystalli Islandici disdiaclastici; the study of the same mineral led Chris tiaan Huygens to discover in 1690 the laws of double refraction, and E. L. Malus in 1808 the polarization of light.
An important property of calcite is the great ease with which it may be cleaved in three directions; the three perfect cleavages are parallel to the faces of the primitive rhombohedron, and the angle between them was determined by W. H. Wollaston in 1812, with the aid of his newly invented reflecting goniometer, to be 55'• The cleavage is of great help in distinguishing calcite from other minerals of similar appearance. The hardness of 3 (it is readily scratched with a knife), the specific gravity of 2.72, and the fact that it effervesces briskly in contact with cold dilute acids are also characters of determinative value.
Crystals of calcite are extremely varied in form, but, as a rule, they may be referred to four distinct habits, namely : rhombo hedral, prismatic, scalenohedral and tabular.
Depending on the habits of the crystals, certain trivial names have been used, such, for example, as "dog-tooth-spar" for the crystals of scalenohedral habit, so common in the Derbyshire lead mines and limestone caverns; "nail-head-spar" for crystals terminated by the obtuse bohedron e, which are common in the lead mines of Alston Moor in Cumberland; "slate-spar" (German Schie f erspat) for tals of tabular habit, and times as thin as paper; spar" for crystals of prismatic habit terminated by the basal plane.
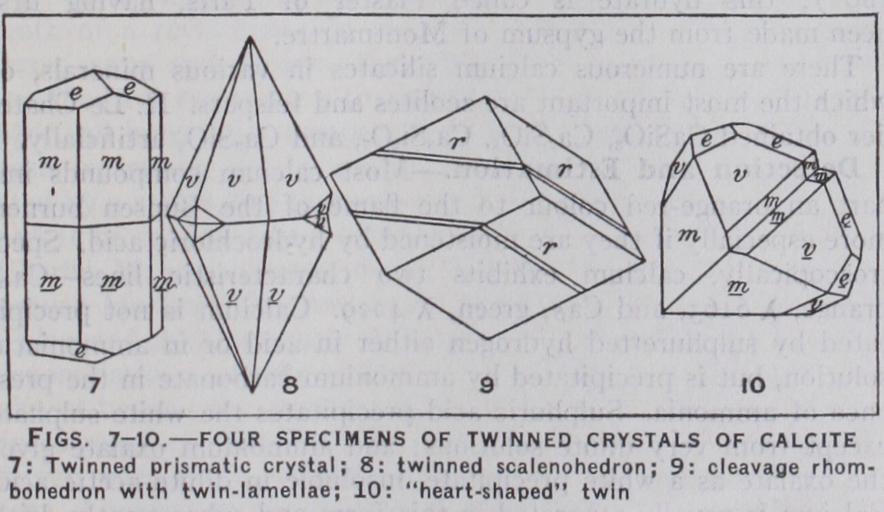
Calcite is also remarkable for the variety and perfection of its twinned crystals. Twinned crys tals, though not of infrequent occurrence, are, however, far less common than simple (un twinned) crystals.
Calcite, when pure, as in the well-known Iceland-spar, is per fectly transparent and colourless. The lustre is vitreous. Owing to the presence of various impuri ties, the transparency and colour may vary considerably. Crystals are often nearly white or colour less, usually with a slight yellow ish tinge. The yellowish colour is in most cases due to the pres ence of iron, but in some cases it has been proved to be due to or ganic matter (such as apocrenic acid) derived from the humus overlying the rocks in which the crystals were formed. An opaque calcite of a grass-green colour, occurring as large cleavage masses in central India and known as hislopite, owes its colour to enclosed "green-earth" (glauconite and celadonite). A stalagmitic calcite of a beautiful purple colour, from Reichelsdorf in Hesse, is coloured by cobalt.
Optically, calcite is uniaxial with negative birefringence, the index of refraction for the ordinary ray being greater than for the extraordinary ray; for sodium-light the former is 1.6585 and the latter 1.4862. The difference, 0.1723, between these two indices gives a measure of the birefringence or double refraction.
Although the double refraction of some other minerals is greater than that of calcite (e.g., for cinnabar it is 0.347, and for calomel 0.683), yet this phenomenon can be best demonstrated in calcite, since it is a mineral obtainable in large pieces of perfect transparency. Owing to the strong double refraction and the con sequent wide separation of the two polarized rays of light travers ing the crystal, an object viewed through a cleavage rhombo hedron of Iceland-spar is seen double, hence the name doubly refracting spar. Iceland-spar is extensively used in the construc tion of Nicol prisms for polariscopes, polarizing microscopes and saccharimeters, and of dichroscopes for testing the pleochroism of gem-stones.
Chemically, calcite has the same composition as the ortho rhombic aragonite (q.v.), these minerals being dimorphous forms of calcium carbonate. Well-crystallized material, such as Iceland spar, usually consists of perfectly pure calcium carbonate, but at other times the calcium may be isomorphously replaced by small amounts of magnesium, barium, strontium, manganese, cobalt, zinc or lead.
Mechanically enclosed impurities are also frequently present, and it is to these that the colour is often due. A remarkable case of enclosed impurities is presented by the so-called Fontainebleau limestone, which consists of crystals of calcite of an acute rhom bohedral form enclosing 5o to 6o% of quartz-sand. Similar crystals, but with the form of an acute hexagonal pyramid, and enclosing 64% of sand, have been found in large quantity over a wide area in South Dakota, Nebraska and Wyoming.
In addition to the varieties of calcite noted above, some others, depending on the state of aggregation of the material, are distin guished. A finely fibrous form is known as "satin-spar," a name also applied to fibrous gypsum : the most typical example of this is the snow-white material, often with a rosy tinge and a pro nounced silky lustre, which occurs in veins in the Carboniferous shales of Alston Moor in Cumberland. Finely scaly varieties with a pearly lustre are known as argentine and aphrite (German Schaumspat) ; soft, earthy and dull white varieties as agaric mineral, rock-milk, rock-meal, etc.—these form a transition to marls, chalk, etc. Of the granular and compact forms numerous varieties are distinguished (see LIMESTONE and MARBLE). In the form of stalactites calcite is of extremely common occurrence.
The modes of occurrence of calcite are very varied. It is a common gangue mineral in metalliferous deposits, and in the form of crystals is often associated with ores of lead, iron, copper and silver. It is a common product of alteration in igneous rocks, and frequently occurs as well-developed crystals in association with zeolites lining the cavities of basaltic and other rocks; and it is said to be a primary constituent in certain igneous rocks. Veins and cavities in limestones are usually lined with crystals of calcite. The wide distribution, under various conditions, of crystallized calcite is readily explained by the solubility of cal cium carbonate in water containing carbon dioxide, and the ease with which the material is again deposited in the crystallized state when the carbon dioxide is liberated by evaporation.
Localities at which beautifully crystallized specimens of calcite are found are extremely numerous. For beauty of crystals and variety of forms the haematite mines of the Egremont district in west Cumberland and the Furness district in north Lancashire are unsurpassed. The lead mines of Alston in Cumberland and of Derbyshire, and the silver mines of Andreasberg in the Harz and Guanajuato in Mexico have yielded many fine specimens. From the zinc mines of Joplin in Missouri enormous crystals of golden-yellow and amethystine colours have been obtained.
The quarry, which since the i 7th century has supplied the famous Iceland-spar, is in a cavity in basalt, the cavity itself measuring 12 by 5yds. in area and about loft. in height. It is situated quite close to the farm Helgustactir, about an hour's ride from the trading station of Eskif jordur on Reydar Fjorctur, on the east coast of Iceland. This cavity when first found was filled with pure crystallized masses and enormous crystals. The crystals measure up to a yard across, and are rhombohedral or scaleno hedral in habit; their faces are usually dull and corroded or coated with stilbite. In recent years much of the material taken out has not been of sufficient transparency for optical purposes; this, with limited supply, has caused a rise in price. (L. J. S.)