Chemistry of Chlorophyll
CHLOROPHYLL, CHEMISTRY OF. Chlorophyll is the green colouring matter of leaves and is present in all growing veg etable cells. Unexpectedly, the pigment responsible for the brown colour of certain algae has been found to be identical with the chlorophyll of land plants. We owe our present knowledge of the chemistry of chlorophyll chiefly to Willstatter.
The chloroplasts of all plants have been found to consist of a colloidal mixture of proteins and other substances with four pig ments, namely, chlorophyll-a, chlorophyll-b, an orange red hydro carbon, called carotin (also occurring in, and responsible for the colour of, carrots), and a yellow pigment, C40H5802, called xanthophyll. It is probable that all these substances are of vital importance in the physiology of the plant, and, indeed, the photo synthesis of starch from carbon dioxide does not proceed in the absence of chlorophyll. Fresh leaves contain about 0.2% of chlorophyll-a, 0.075% of chlorophyll-b, 0.015% of carotin and 0.03% of xanthophyll. In order to obtain crude chlorophyll, dried powdered leaves (best of the stinging nettle) are extracted with ether, and the amorphous chlorophyll so obtained has been found to have the composition Analytical data, however, do not establish with certainty the true composition of such a complex substance, and in the light of later work (especially the synthesis of aetiophyllin) it may be that the formulae need to be corrected by the addition of one carbon atom. The compo sitions given here are those employed by Willstatter in describing his experiments. The discovery that magnesium is an essential part of the chlorophyll molecule was an event of outstanding im portance and interest ; the metal is bound in a complex condition, very much as iron is in the blood pigments. In fact a complex degradation product of the blood pigment can actually be con verted into a complex degradation product of chlorophyll by introducing magnesium into the molecule. It is highly significant that chlorophyll and the blood pigment are so closely related.
If alcohol is used instead of ether for the extraction of chloro phyll, the pigment is obtained in a crystalline modification, but interaction with the alcohol has occurred as the result of the intervention of a specific enzyme termed chlorophyllase. Actually the phytyl group (see below) is replaced by the ethyl group. In 1864 Stokes demonstrated that chlorophyll is a mixture, but it was not until 1912 that the components of the mixture were separated. If a solution of crude chlorophyll in light petroleum is shaken with aqueous methyl alcohol, chlorophyll-a remains in the petroleum whereas chlorophyll-b is found in the aqueous layer. Both substances may be crystallized ultimately after a tedious series of fractionations. Chlorophyll-a is bluish black and gives greenish-blue solution ; it contains H, more and 0 less than chlorophyll-b, which is a greenish-black substance giving green solutions.
All true chlorophylls, amorphous, and a and b (material from over 200 sources was investigated) yield on treatment with alco holic alkalies about 30% of their weight of phytol together with a molecular proportion of methyl alcohol. Phytol is an unsatu rated alcohol, C (CH,) : C . or which has not yet been fully investigated but evidently bears some relation to the terpene family. The methyl alcohol and phytol are bound in groups of the form —CO—OR because the chloro phylls are converted by this treatment into the chlorophyllins which are carboxylic acids. Thus the constitution of chlorophyll-a can be expanded to (C3,H300N,Mg) (COOC.1-1.) and chlorophyllin-a is the corresponding dicarboxylic acid. The pres ence of a third carboxyl group hidden in the form —CO—N: has been detected and the main line of degradation of the chlorophyll molecule has been the step-wise elimination of these carboxyl groups. For this purpose the graded action of alkalies at tempera tures up to 24o° has been employed, and it is noteworthy that the magnesium-containing complex in the molecule is not decom posed in the course of such treatment. Many of the intermediate products are brilliantly coloured and exhibit intense fluorescence. The action of acids at any stage causes the elimination of mag nesium, but this can usually be re-introduced by means of magnesia or magnesium methyl iodide. It would be beyond the scope of this article to give a full account of the degradation of chlorophyll, but the stages from chlorophyll-a to aetioporphyrin may be indicated. Alcoholysis and hydrolysis by chlorophyllase yields chlorophyllide-a (C32H300N1Mg) (CO,Me) (CO,H) which with cold alkali gives chlorophyllin-a (C,,H,,ON,Mg) Alkali at 140°C yields glaucophyllin (C31H,,N,Mg) (CO2H),, and at 165°C this in its turn yields the isomeric rhodophyllin. The second carboxyl is eliminated by alkali treatment at 200°C, giving pyrrophyllin whilst the last carboxyl group is removed by heating with soda lime in small quantities at a time. The product is aetiophyllin, Acids acting on this yield aetioporphyrin, C,,H,,N,, which is identical with the degra dation product of the blood pigment haemin. The action of cold alkali and then alkali at 200°C also converts chlorophyll-b into pyrrophyllin.
A more intimate knowledge of the constitution of chlorophyll has resulted from the study of the degradation of the derivatives of the colouring matter by reduction with hydriodic acid and phosphonium iodide and by oxidation. The former method gave alkylated pyrroles identical with those obtained similarly from the blood pigment and from bilirubin ; the latter method gave such substances as methylethylmaleinimide and haematic acid. Indeed, the chemistry of chlorophyll and of the blood pigments is so closely related that information derived from the study of one of these groups has had repercussions in the other.
A highly remarkable synthesis effected by H. Fischer and J. Klarer in 1926 enables us to make a short cut and deduce a probable expression for the structure of aetioporphyrin. Cryp topyrrole, 2 :4-dimethyl-3-ethylpyrrole, is one of the reduction products of the pigments under notice and had been previously synthesized by unambiguous methods. When it is brominated in cold acetic acid solution it gives a substance, which is converted into Willstatter's aetioporphyrin by the action of strong sulphuric acid. This seems to prove that aetioporphyrin is and the interpretation suggested leads to the following constitution for aetioporphyrin :— The aetioporphyrin was identified with the natural product by a comparison of solubility, crystallographic characters and absorp tion spectrum. It gives aetiophyllin on treatment with magne sium methyl iodide. This substance is therefore N,Mg and the corrected composition of chlorophyll-a is accordingly (CO,CH,) or Chloro phyll-b would then be C.H ,N,O,Mg. It is known from the oxi dation products that the carboxyl groups are all situated in the ter minal positions of groups, that is, in groups, but we have no information as to the relative position of the phytol and methyl alcohol residues or as to which of the three carboxyl groups is bound up in the —CO—N: structure.
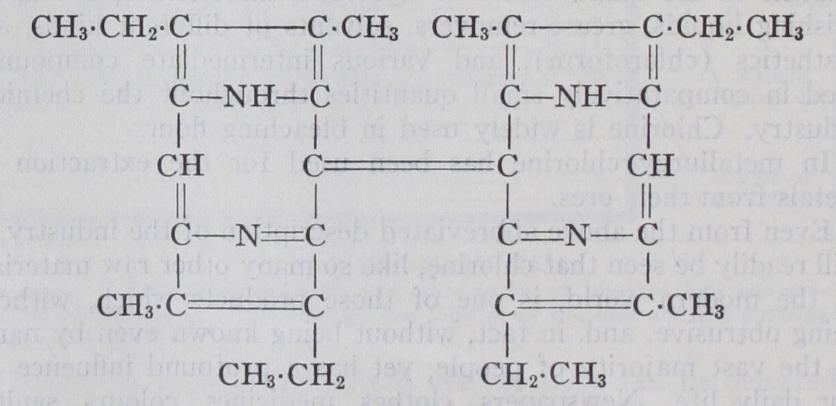
It will be noted, in the degradation of chlorophyll, that one of the carboxyl groups is much more easily eliminated than the other two, and we may well imagine that an unstable progenitor of the pigment contained still one more carboxyl group, so that each in aetiophyllin was originally If this were assumed then the suggestive fact emerges that the molecule could have been constructed in nature by the conden sation of four identical monohydric alcohol chains with ammonia. Thus chlorophyllin could be the result of reactions summarized in the equation It becomes evident that chlorophyll is derived in nature from the sugars which it is itself designed to produce through photo synthesis.
Willstatter and Stoll have described their investigations in a monograph "Untersuchungen fiber Chlorophyll" (Springer, Ber lin, . ROB.)
