Chlorine
CHLORINE, a gaseous chemical element of the halogen group, taking its name from the colour, greenish-yellow (Gr. XXwpos) ; symbol Cl, atomic number 17, atomic weight 35.457, isotopes 35, 37. It was discovered in 1774 by Scheele, who called it dephlogisticated muriatic acid; about 1785, C. L. Berthollet, regarding it as being a compound of hydrochloric acid and oxygen, termed it oxygenized muriatic acid. This view was generally held until about 1810–II, when Sir H. Davy showed definitely that it was an element, and gave it the name which it now bears.
Chlorine is never found in nature in the uncombined condition, but in combination with the alkali metals it occurs widely distrib uted in the form of rock-salt (sodium chloride) ; as sylvine and carnallite, at Stassfurt; and to a smaller extent in various other minerals such as matlockite and horn-mercury. In the form of alkaline chlorides it is found in sea-water and various spring waters, and in the tissues of animals and plants ; while, as hydro chloric acid it is found in volcanic gases.
The preparation of chlorine on the small scale depends on the oxidation of hydrochloric acid ; the usual oxidizing agent is man ganese dioxide, which, when heated with concentrated hydrochloric acid, forms manganese chloride, water and chlorine The manganese dioxide may be re placed by various other substances, such as red lead, lead dioxide, potassium bichromate, and potassium permanganate. Instead of heating hydrochloric acid with manganese dioxide, use is fre quently made of a mixture of common salt and manganese dioxide, to which concentrated sulphuric acid is added and the mixture is then (For electrolytic and other industrial methods of preparation, see CHLORINE: In Industry.) Chlorine is a gas of a greenish-yellow colour, and possesses a characteristic unpleasant and suffocating smell. It can be liquefied at —34°C under atmospheric pressure, and at —102°C it solidifies and crystallizes. Its specific heat at constant pressure is and at constant volume 0•08731, and its refractive index 1.00077 2, whilst in the liquid condition the refractive index is 1.367. The density is 2.4885 (air=1) . Its critical temperature is 146°C and its critical pressure 93•5 atm.; it is not appreciably dissociated at 15oo°C, but at 2350°C 5o% of the molecules are broken down to atoms. Liquid and solid chlorine are both yellow in colour. The gas must be collected either by downward displacement, since it is soluble in water and also attacks mercury ; or over a saturated salt solution, in which it is only slightly soluble. At ordinary tempera tures it unites directly with many other elements ; thus with hydro gen, combination takes place in direct sunlight with explosive vio lence; arsenic, antimony, thin copper foil and phosphorus take fire in an atmosphere of chlorine, forming the corresponding chlor ides. Many compounds containing hydrogen are readily decom posed by the gas ; for example, a piece of paper dipped in turpen tine inflames in an atmosphere of chlorine, producing hydrochloric acid and a copious deposit of soot ; a lighted taper burns in chlor ine with a dull smoky flame. The solution of chlorine in water, when freshly prepared, possesses a yellow colour, but on keeping becomes colourless, on account of its decomposition into hydro chloric acid and oxygen. It is on this property that its bleaching and disinfecting power depends (see BLEACHING). Water satu rated with chlorine at o°C deposits crystals of a hydrate which is readily decomposed at a higher temperature into its con stituents. Chlorine hydrate has an historical importance, as by sealing it up in a bent tube, and heating the end containing the hydrate, whilst the other limb of the tube was enclosed in a freez ing mixture, M. Faraday was able to obtain liquid chlorine.
Chlorine finds an extensive use in organic chemistry as a substi tuting and oxidizing agent, as well as for the preparation of addi tion compounds. For purposes of substitution, the free element, as a rule, only works slowly on saturated compounds, but the reac tion may be accelerated by the action of sunlight or on warming, or by using a "carrier." In these latter cases the reaction may pro ceed in different directions; thus, with the aromatic hydrocarbons, chlorine in the cold or in the presence of a carrier substitutes in the benzene nucleus, but in the presence of sunlight or on warming, substitution takes place in the side chain. Iodine, antimony tri chloride, molybdenum pentachloride, ferric chloride, ferric oxide, antimony, tin, stannic oxide and ferrous sulphate have all been used as chlorine carriers.
Hydrochloric Acid.—Chlorine combines with hydrogen to form hydrochloric acid, HC1, the only known compound of these two elements. The acid itself was first obtained by J. R. Glauber in about 1648, but J. Priestley in 1772 was the first to isolate it in the gaseous condition, and Sir H. Davy in 1810 showed that it con tained hydrogen and chlorine only, as up to that time it was con sidered to contain oxygen. It may be prepared by the direct union of its constituents, and with the displacement of the Leblanc soda process by the ammonia-soda and electrolytic-soda processes the production of synthetic hydrochloric acid has acquired industrial importance. On the large scale and also for the preparation of small quantities it is made by the decomposition of salt by means of concentrated sulphuric acid, (See ALKALI MANUFACTURE.) The commercial acid is usually yellow in colour and contains many impurities, such as traces of arsenic, sulphuric acid, chlorine, ferric chloride and sulphurous acid.
It is a colourless gas, which can be condensed by cold and pressure to a liquid boiling at —83.7°C, and can also be solidified, the solid melting at —112.5°C. Its critical temperature is 52.3°C, and its critical pressure is 86 atmospheres. The gas fumes strongly in moist air, and it is rapidly dissolved by water, one volume of water at o°C absorbing 503 volumes of the gas. The gas does not obey Henry's law, that is, its solubility in water is not proportional to its pressure. It is one of the "strong" acids, being ionized to the extent of about 91.4% in decinormal solution according to conduc tivity and cryoscopic methods (see SOLUTION). The strongest aqueous solution of hydrochloric acid at 15°C contains 42.9% of the acid, and has a specific gravity of I.212. On being boiled at ordinary pressure, solutions stronger than 2o.24% lose HCI, and weaker solutions lose water until a mixture of this composition is obtained; this boils constantly at II o° and has a specific gravity of I • I o, but it is not a definite hydrate because its composition is slightly different if it is produced by evaporation at higher or lower pressures. Perfectly dry hydrochloric acid gas has no action on metals, but in aqueous solution it dissolves many of them with evolution of hydrogen and formation of chlorides.
Chlorides.
The salts of hydrochloric acid, known as chlorides, can, in most cases, be prepared by dissolving either the metal, its hydroxide, oxide, or carbonate in the acid ; or by heating the metal in a current of chlorine, or by precipitation. The majority of the metallic chlorides are solids (stannic chloride, titanic chloride and antimony pentachloride are liquids) which readily volatilize on heating. Many are readily soluble in water, the chief exceptions being silver chloride, mercurous chloride, cuprous chloride and palladious chloride which are insoluble in water, and thallous chloride and lead chloride which are only slightly soluble in cold water, but are readily soluble in hot water. Bismuth and antimony chlorides are decomposed by water with production of oxychlor ides, whilst titanium tetrachloride yields titanic acid under the same conditions. All the metallic chlorides, with the exception of those of the alkali and alkaline earth metals, are reduced either to the metallic condition or to that of a lower chloride on heating in a current of hydrogen ; most are decomposed by concentrated sul phuric acid. They can be distinguished from the corresponding bromides and iodides by the fact that on distillation with a mix ture of potassium bichromate and concentrated sulphuric acid they yield chromium oxychloride, whereas bromides and iodides by the same treatment give bromine and iodine respectively. Some metallic chlorides readily form double chlorides, the most impor tant of these double salts being the platinichlorides (chloroplati nates) of the alkali metals. The chlorides of the non-metallic ele ments are usually volatile fuming liquids of low boiling-point, which can be distilled without decomposition and are decomposed by water. Hydrochloric acid and its metallic salts can be recog nized by the formation of insoluble silver chloride, on adding silver nitrate to their nitric acid solution, and also by the formation of chromium oxychloride (see above). Chlorides can be estimated quantitatively by conversion into silver chloride, or if in the form of alkaline chlorides (in the absence of other metals, and of any free acids) by titration with standard silver nitrate solution, using potassium chromate as an indicator (q.v.).
Oxides.
Chlorine and oxygen do not combine directly, but compounds can be obtained indirectly. Three oxides are known : chlorine monoxide, chlorine peroxide, and chlorine heptoxide, Chlorine monoxide results on passing chlorine over dry precipi tated mercuric oxide at o°C. It is a pale yellow gas which can be condensed, on cooling, to a dark-coloured liquid boiling at 19°C. It is extremely unstable, decomposing with extreme violence on the slightest shock or disturbance, or on exposure to sunlight. It is readily soluble in water, with which it combines to form hypo chlorous acid. Sulphur, phosphorus, carbon compounds, and the alkali metals react violently with the gas, taking fire with explosive decomposition.Chlorine peroxide was first obtained by Sir H. Davy in 1815 by the action of concentrated sulphuric acid on potassium chlorate : A mixture of chlorine peroxide and chlorine (which Davy called "euchlorine") is obtained by the action of hydrochloric acid on potassium chlo rate, and similarly, on warming a mixture of potassium chlorate and oxalic acid to 7o°C on the water bath, a mixture of chlorine peroxide and carbon dioxide is obtained. Chlorine peroxide must be collected by displacement, as it is soluble in water and readily attacks mercury. It is a heavy gas of a deep yellow colour and possesses an unpleasant smell. It can be liquefied, the liquid boil ing at 9.9°C, and on further cooling it solidifies to orange crystals at —79°C. It is highly explosive, being resolved into its constitu ents by influence of light, by warming, or by shock. It is a very powerful oxidant ; a mixture of potassium chlorate and sugar spon taneously inflames when touched with a drop of concentrated sul phuric acid or even on rubbing, the chlorine peroxide liberated setting fire to the sugar, which goes on burning.
Chlorine heptoxide was obtained by A. Michael (1900, 1901) by slowly adding perchloric acid to phosphoric oxide below —1 o° C ; the mixture is allowed to stand for a day and then gently warmed, when the oxide distils over as a colourless very volatile oil of boil ing-point 82°C. It turns to a greenish-yellow colour in two or three days and gives off a greenish gas ; it explodes violently on percussion or in contact with a flame, and is gradually converted into perchloric acid by the action of water. On the addition of iodine to this oxide, chlorine is liberated and a white substance is produced, which decomposes, on heating to 38o°C, into iodine and oxygen ; bromine is without action.
Oxy-acids.
Several oxy-acids of chlorine are known, namely, hypochlorous acid, HC10, chlorous acid, chloric acid, and perchloric acid, Hypochlorous acid is formed when chlorine monoxide dissolves in water, and can be prepared (in dilute solution) by passing chlorine through water containing precipitated mercuric oxide in suspension, or best by passing car bon dioxide through a suspension of bleaching powder in water. Precipitated calcium carbonate may be used in place of the mer curic oxide, or a hypochlorite may be decomposed by a dilute min eral acid and the resulting solution distilled under diminished pressure. For this purpose a filtered solution of bleaching-powder and a very dilute solution of nitric acid may he employed. The acid is only known in aqueous solution, and only dilute solutions can be distilled without decomposition. The solution has a pale yellow colour, and is a strong oxidizing and bleaching agent ; it is readily decomposed by hydrochloric acid, with evolution of oxy gen. The salts of this acid are known as hypochlorites, and like the acid itself are very unstable, so that it is almost impossible to obtain them pure. A solution of sodium hypochlorite (Eau de Javelle), which can be prepared by passing chlorine into a cold aqueous solution of caustic soda, has been extensively used for bleaching purposes. One of the most important derivatives of hypochlorous acid is bleaching powder. Sodium hypochlorite can be prepared by the electrolysis of brine solution in the presence of carbon electrodes, having no diaphragm in the electrolytic cell, and mixing the anode and cathode products by agitating the liquid. The temperature should be kept at about 15°C, and the concentra tion of the hypochlorite produced must not be allowed to become too great, in order to prevent reduction taking place at the cath ode. Its solutions are used in preservatives and antiseptics, but on keeping or warming they decompose to chloride and chlorate.Chlorous acid has been prepared in solution by adding sulphuric acid in theoretical proportion to a solution of the barium salt ; its solutions readily decompose to hypochlorous and chloric acids. The sodium salt is prepared by the action of sodium peroxide on a solution of chlorine peroxide : The silver and lead salts are unstable, being decomposed with explosive violence at 1 oo° C. On adding a caustic alkali solution to one of chlorine peroxide, a mixture of a chlorite and a chlorate is obtained.
Chloric acid was discovered in 1786 by C. L. Berthollet, and is best prepared by decomposing barium chlorate with the calculated amount of dilute sulphuric acid. The aqueous solution can be con centrated in vacuo over sulphuric acid until it contains 4o% of chloric acid. Further concentration leads to decomposition, with evolution of oxygen and formation of perchloric acid. The concen trated solution is a powerful oxidizing agent ; organic matter being oxidized so rapidly that it frequently inflames. Hydrochloric acid, sulphuretted hydrogen and sulphurous acid are rapidly oxidized by chloric acid. The salts of this acid are known as chlorates (q.v.).
Perchloric acid is best prepared by distilling potassium per chlorate with concentrated sulphuric acid under diminished pres sure. Perchloric acid distils over at first, but if the distillation be continued a white crystalline mass of hydrated perchloric acid, passes over ; this is due to the decomposition of some of the acid into water and lower oxides of chlorine, the water produced then combining with the pure acid to produce the hydrated form (H. E. Roscoe). This solid, on redistillation, gives the pure acid, which is a liquid boiling at 39°C (under a pres sure of 56mm.), melting at —112°C, and of specific gravity ° 1-7641 22 • The crystalline hydrate melts at 50°C. A constant 4boiling solution (b.p. 203°C under 76omm. pressure) of 71.6% content results as in the case of hydrochloric acid (see above). The pure acid decomposes slowly on standing, but is stable in dilute aqueous solution. It is a very powerful oxidizing agent ; wood and paper in contact with the acid inflame with explosive violence. In contact with the skin it produces painful wounds. It may be distinguished from chloric acid by the fact that it does not give chlorine peroxide when treated with concentrated sul phuric acid, and that it is not reduced by sulphurous acid. The salts of the acid are known as the perchlorates, and are all soluble in water; the potassium and rubidium salts, however, are only soluble to a slight extent, and methods for the estimation of these metals are based on this fact. Potassium perchlorate, KC10,, can be obtained by carefully heating the chlorate until it first melts and then nearly all solidifies again. The fused mass is then ex tracted with water to remove potassium chloride, and warmed with hydrochloric acid to remove unaltered chlorate, and finally ex tracted with water again, when a residue of practically pure per chlorate is obtained. The alkaline perchlorates are isomorphous with the corresponding permanganates.
As an industrial product of increasing importance chlorine is closely connected with alkali and the manufacturing industries are, in practice, combined. It has been pointed out in another article (see ALKALI) that the manufacture of chlorine has under gone a complete revolution in recent years. During the ascend ancy of the Le Blanc soda process, it was necessary to produce chlorine in order to reduce the great surplus of unwanted hydro chloric acid, which served as the raw material. Nowadays the reverse is the case, and the production of caustic soda, by the electrolytic process, is limited by the extent to which the accom panying chlorine can be utilized.
Chemically speaking, chlorine can be produced in several ways; i.e., from hydrochloric acid with the aid of "oxidizing" agents; from salt by the action of sulphuric acid and an oxidizing agent combined; or by the so-called "electrolytic method," in which an electric current is passed through a solution of common salt in water. The two former methods are now obsolete ; the Deacon and Weldon processes were examples of the oxidation of hydro chloric acid, and ceased to be operated when the Le Blanc soda process was finally superseded by Solvay's ammonia-soda method. The electrolytic process is the source of all the chlorine now pro duced on the large scale. The action of the electric current on brine is to produce chlorine gas, hydrogen gas and a solution of caustic soda simultaneously. The caustic soda is concentrated and solidified; the hydrogen is either allowed to go to waste or is used for the synthesis of ammonia (with nitrogen) or of hydrochloric acid (with chlorine) ; the chlorine gas is piped off and utilized for making either liquid chlorine, in which there is now a large trade; bleaching powder; hydrochloric acid; calcium and sodium hypo chlorites ; sulphur-chlorine compounds; chlorinated organic com pounds; or metallic chlorides.
The raw materials of the industry are salt and coal; the former as the actual source of the chlorine, and the latter to provide the energy for removing it from its combination with the sodium. The industry therefore tends to concentrate in districts where brine is available, and where cheap energy exists, either as coal or water power. Where water-power is to be had it may prove economical to site the works there and bring salt from a distance. In general, and as in the case of alkali, the chlorine industry is one in which it is cheaper to build the plant close to the raw materials rather than close to the markets in which the products are to be sold.
England, Germany and North America are the chief producing countries, and Italy is developing rapidly. In the two former countries the industry has long been well-established, but in Can ada and the United States, with their great deposits of salt, and unrivalled sources of cheap waterpower, it is comparatively of recent development ; it has nevertheless already reached such di mensions that practically the whole of the requirements of these countries can now be satisfied without imports. Of recent years Italy also has gone ahead in this matter, largely owing to the rise of the artificial silk industry there; this requires large quantities of caustic soda which, taken together with a well-organized system of water-power, provides a good basis for electrolytic chlorine-soda manufacture.
England and Germany are the chief exporting countries. The total production of chlorine and chlorine compounds amounts to 300,00o tons per annum. The largest chlorine manufacturing plant in /928 was situated at South Charleston, W.Va., with a capacity of one hundred tons per day.
Manufacture of Chlorine.
The electrolytic process is now adays the only source of chlorine on a large scale. It is neverthe less of interest, both from a historical as well as a chemical point of view, to give some account of the older methods.C. W. Scheele, who discovered chlorine in 1774, made use of manganese dioxide, in the form of the natural mineral pyrolusite, to oxidize hydrochloric acid :— This reaction must be promoted by heating the mixture, although even then only a part of the hydrochloric acid is oxidized. Owing to strongly corrosive action of both hydrochloric acid and moist chlorine, no metal could be employed in the construction of ves sels, which were made of stone slabs. The process was very costly, as much of the acid and all the manganese was wasted, and the waste liquor from the vessels was of a most noxious kind.
The chemistry of the process is not quite so simple as the fore going description indicates, but sufficient has been said to give a general outline of it. Of the hydrochloric acid originally employed only some 35% is actually converted to chlorine, the remainder being lost as calcium chloride.
The second well-known process, due to Henry Deacon working from /868 onwards, is also based on the oxidation of hydrochloric acid, but using atmospheric air in place of manganese dioxide. Deacon worked on the direct reaction This reaction is under ordinary circumstances so slow as to be quite useless for practical purposes. If, however, a "catalyst" is employed at the proper temperature, the process proceeds at a very much greater rate, and can even be carried out as a continu ous operation.
The catalyst that Deacon used was cupric chloride : if pieces of porous clay be soaked in a solution of this salt, dried, and heated to about 450° C. and the temperature maintained there or slightly higher, they are found to have the property of producing chlorine and water continuously from a stream of mixed hydrochloric acid gas and air passed over them. On a large scale about 6o% of the acid can thus be decomposed. The air-acid mixture was taken directly from the pan in which salt cake (q.v.) had been pre pared, after cooling to condense out water and reheating to the temperature necessary for the catalytic reaction.
The Electrolytic Method.
The Deacon process made cheap er chlorine than the Weldon process : but it is evident that in both cases the plant was complicated, costly and difficult to manipulate. The contrast between these older methods and the modern electro lytic process is very marked.The various types of plant employed nowadays all depend upon the decomposition of the molecules of salt by means of the ap plied energy of the electric current. Faraday discovered that equal quantities of electric energy will liberate elements from their compounds in amounts proportional to their equivalent weights: for example, 23 grams of sodium, 35.5 grams of chlorine, io7 grams of silver, 3 r • 5 grams of copper, are all set free from solu tions of their salts, or from the fused salts, under appropriate conditions by the same quantity of electric energy, viz., 96,540 coulombs. The degree to which this decomposition is brought about is a function solely of the amount of electric energy applied, and does not depend on the voltage, or electrical pressure. At the same time, owing to considerations which cannot be enlarged upon here, it is found that a certain minimum voltage is required before actual liberation of the elements takes place; the voltage actually used in practice depends upon the type of "cell" in which the electrolysis is conducted, and may vary from 3.5 to 7 volts.
A very large number of cells have been devised to work this process; but, neglecting the Acker process, which used fused sodium chloride, only three principal types are now in use; viz., the mercury cell, the diaphragm cell and the "belljar" cell.
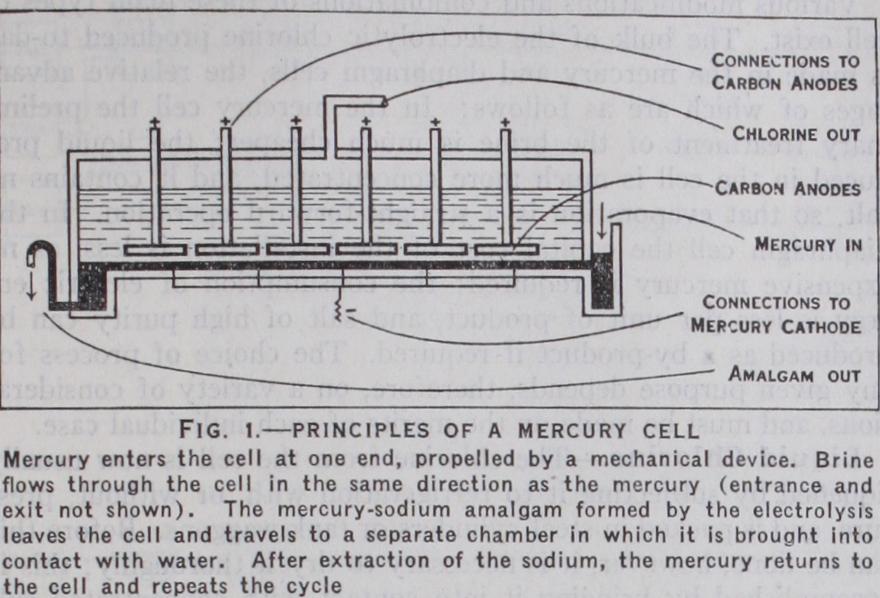
In each of these types a solution of sodium chloride (brine) is used ; the points at which the electric current is introduced into the solution are called respectively the "anode" and "cathode." The mercury cell (fig. i) has the form of a rectangular hori zontal box; saturated brine, i.e., brine containing as much salt as possible, is fed into the cell at one end and overflows in the par tially spent condition at the other. Into this brine dip anodes of graphitic carbon; on the bottom of the cell is a layer of mercury which forms the cathode. The action of the current liberates chlorine upon the graphite anodes in the form of a gas, which is conveyed away by pipes for sub sequent use ; at the cathode so dium is deposited and at once forms an amalgam with the mer cury. This amalgam is caused to flow into another compartment, where it is brought into contact with water, the effect of which is to extract the sodium with f or mation of a dilute solution of caustic soda and hydrogen gas; the disposal of the latter has already been touched upon. After the removal of the sodium the mercury returns to the first com partment. This type of cell, which is now largely employed, was devised by Castner in the United States, and by Kellner in Austria. In the original form the movement of the mercury was occasioned by rocking the cell on a pivot ; in more modern plant the mercury is propelled by mechanical means, the cell itself being stationary. The alkali thus produced is of high purity, and the "current efficiency" or yield per unit of current good; but the plant is expensive to erect and the mercury costly.
The diaphragm type of cell (fig. 2) uses no mercury which can be moved from one compartment to another, and it is therefore necessary to provide some means whereby the sodium and the chlorine may be collected separately.
To effect this, the cell, which, in the form chosen for descrip tion here, takes the shape of a cylinder on end, is divided into two annular compartments by a ring or diaphragm of asbestos fibre, supported by a perforated metal sheet or wire screen on the outer side. This iron gauze forms the cathode at which the so dium separates. The anode is formed by graphitic carbon sticks suspended from the top of the cell and hanging vertically in the inner compartment of the cell. This compartment is fed with purified brine. Purification of the brine from the calcium and magnesium compounds, which occur naturally in it, is necessary on account of the subsequent blocking and deterioration of the asbestos diaphragm which would otherwise occur: the purifica tion is carried out by precipitating the calcium as carbonate and the magnesium as hydroxide by treatment with sodium carbon ate and caustic soda. As the asbestos diaphragm is permeable to liquid some brine runs through it, so that the metal sheet on the outside is continually wet. On passing the current through the cell, chlorine is liberated at the anode as in the mercury cell, and sodium momentarily at the metal cathode, where it at once re acts with the water of the brine which has soaked through the asbestos, and forms a solution of caustic soda, with simultaneous evolution of hydrogen gas. The liquor thus formed contains a large proportion of undecomposed salt, which is recovered in solid form during the subsequent evaporation for the production of solid caustic soda. Not only does this cause some complication in the method adopted for evaporation, but the resulting caustic soda cannot be obtained free from salt, and contains usually about 2% of it.
In the Belljar type (fig. 3) the anode is surrounded by an in verted bell of material, which does not conduct electricity, placed in a tank in which the cathode is suspended. Brine is fed into the interior of the bell and the chlorine there produced is piped off as before. The caustic soda formed at the cathode sinks, by virtue of its greater density, through the brine and is drawn off through a pipe. The liquor produced is similar to that formed in the diaphragm process.
Various modifications and combinations of these main types of cell exist. The bulk of the electrolytic chlorine produced to-day is made in the mercury and diaphragm cells, the relative advan tages of which are as follows : In the mercury cell the prelim inary treatment of the brine is much cheaper; the liquid pro duced in the cell is much more concentrated, and it contains no salt, so that evaporation is a straight-forward operation. In the diaphragm cell the capital cost of the installation is less, as no expensive mercury is required ; the consumption of electric en ergy is less per unit of product, and salt of high purity can be produced as a by-product if required. The choice of process for any given purpose depends, therefore, on a variety of considera tions, and must be made on the merits of each individual case.
