Chromosphere
CHROMOSPHERE is the name which was given by Sir Norman Lockyer in i868 (at the suggestion of Sharpey, then secretary of the Royal Society) to the layer of the sun's atmos phere, just outside the photosphere, which is observed visually when the sun is totally eclipsed and is spectroscopically observ able at other times. Observations of the chromosphere had in fact been made before 1868. Thus Young (The Sun, 1882) men tions that Capt. Stannyan, in a report on the eclipse of 1706 observed by him at Berne, noticed "a blood-red streak of light visible for six or seven seconds upon the western limb," just be fore the emergence of the sun. It was observed also by Halley in 1715, and by Arago, Airy, Secchi and others at eclipses in the i 9th century. Attention was however directed mostly at that time to solar prominences, and the chromosphere escaped serious study. In 1868 Janssen and Lockyer independently discovered that solar prominences, hitherto only observed at total eclipses, could be seen in full daylight by means of the spectroscope—the principle being that the white light from the sky surrounding the sun's disc was weakened by dispersion on passing through the spectroscope, whilst any monochromatic constituent of the light from the prominences passed through undispersed and gave rise to a bright line. Lockyer shortly afterwards (Nov. 5, 1868 ) noticed that the prominences jutted out from a continuous spheri cal envelope surrounding the sun. This envelope was at all times visible in the red (C or Ha) and blue (F or Hsi) lines of hydrogen and in the yellow line of the then unidentified element he lium. It was to this continuous envelope that Lockyer gave the name chromosphere, in reference to the colour effects seen in the spectroscope.
At a time of total solar eclipse, at the moment of second contact, the chromosphere becomes visible as a thin red crescent, some io sec. in thickness, on the east side of the disc. The advancing moon rapidly covers up the layer, which reappears at the western limb just before third contact. The red colour is due to the visual dominance of the Ha line of hydrogen.
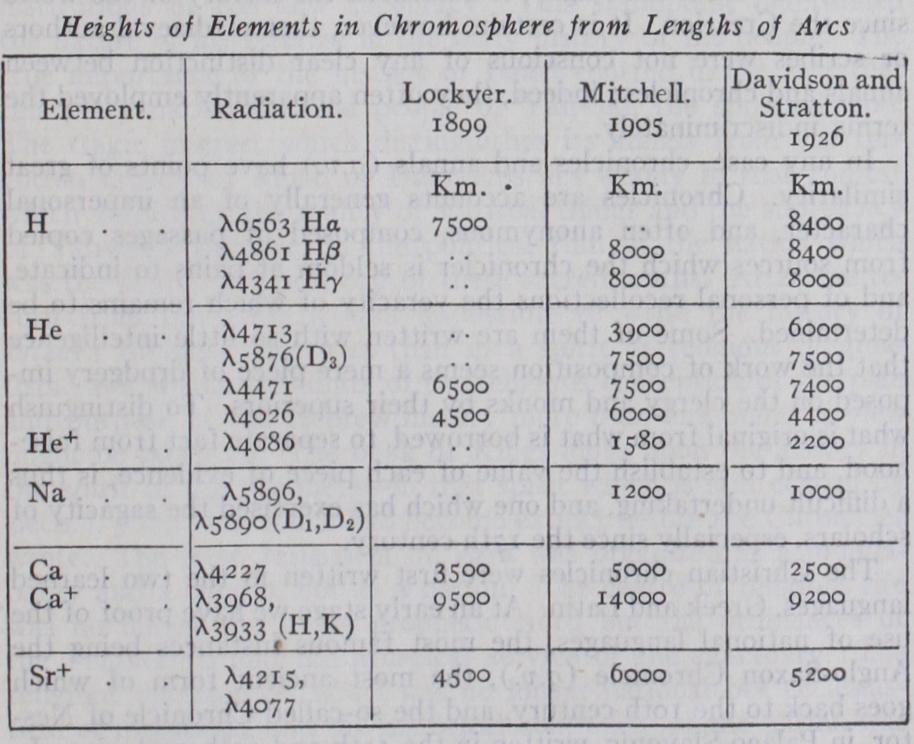
Astronomers soon recognized that in the chromosphere they must be viewing tangentially the upper layers of the same gases which, when projected against the bright disc, produce the ordinary Fraunhofer absorption spectrum of sunlight, and it was natural to look for confirmation of this. The complete spectrum of the light from the chromosphere might be expected to corre spond with the Fraunhofer spectrum, a bright line in the chromo spheric spectrum corresponding to each dark line in the Fraun hofer spectrum. This was first verified by C. A. Young at the total eclipse of Dec. 22, 187o. Placing the slit of his spectro scope tangential to the advancing limb of the moon, he saw the Fraunhofer spectrum suddenly replaced by a spectrum of bright lines which lasted only two or three seconds. This spectrum was hence called by Young the "flash spectrum." In Young's own words, "as the moon advances, making narrower and narrower the remaining sickle of the sun's disc, the dark lines of the spec trum remain sensibly unchanged though becoming somewhat less intense. A few however begin to fade out, and some even turn partially bright a minute or two before totality begins. But the moment the sun is hidden, through the whole length of the spec trum, in the red, the green, the violet, the bright lines flash out by hundreds and thousands, almost startlingly; as suddenly as stars from a bursting rocket, and as evanescent, for the whole thing is over in two or three seconds." Photography was first successfully applied to the photography of the flash spectrum by A. Fowler and W. Shackleton at the eclipse of April 16, 1893. The flash spectrum may be observed by the use of a tangential slit, as in Young's original observation. But the crescent of atmosphere left exposed by the moon's disc during the period of the flash is so thin that it behaves as a crescent-shaped slit. A direct photograph of the flash spectrum with a prismatic camera without slit reveals a series of crescents, one corresponding to each bright line in the flash spectrum. (See illustration under article ECLIPSE.) Each gives an image of the chromosphere and prominences in the corresponding radiation. The thicker the atmosphere as viewed in the light of a particular radiation, the longer will be the arc in the photograph, and it is a matter of simple geometry to deduce the height of the chromo sphere in any given radiation from a measurement of the dis tance from cusp to cusp, using the known diameters of the sun and moon. The following table shows chromospheric heights de rived in this way by Lockyer (eclipse of 1898), Mitchell (1905) and Davidson and Stratton (1926).
As regards wave-length, the flash spectrum is an almost exact copy of the Fraunhofer spectrum. The Balmer series of hydrogen extends however much further (Stratton and Davidson at Su matra, in 1926, observed 36 members of the series) ; also helium is prominent in the flash, whilst it only appears fitfully and faint ly in the Fraunhofer spectrum. But the intensities of the lines in the flash are markedly different from the intensities in the Fraun hofer spectrum. Lines faint in the Fraunhofer spectrum give rise to short intense arcs in the flash ; this is easily explained since the faint lines in the Fraunhofer spectrum are probably produced by low-lying vapours at a high temperature, which accordingly shine brightly when viewed tangentially. But in addition many lines are enormously increased in intensity in the flash. It was pointed out by Lockyer that such lines are almost invariably "en hanced" lines, i.e., lines which are relatively strengthened from arc spectra to spark spectra. For example the D-lines of sodium (arc lines) are relatively inconspicuous in the flash; the blue line X42 2 7 of calcium is much less intense and extends to a smaller height than the H and K lines ; the enhanced lines of iron, scandium, titanium and chromium are all strengthened in the flash, and usually extend to greater heights than the unenhanced lines. The flash spectrum, in fact, resembles in many ways the spectrum of stars much hotter than the sun. This was for many years a difficulty, for it was hard to believe that the high-level chromosphere could be hotter than the low-level reversing layer.
The explanation was given by Megnad Saha in 1920. En hanced lines are now known to be due to the ionized atom, i.e., the atom which has lost one or more electrons, and Saha showed that ionization was promoted not only, as in Lockyer's experi ments, by high temperature but also by low pressure. At high temperatures the process of the dissociation of an atom into an electron and a positive ion goes on of its own accord, according to the reversible equation A until a balance is obtained between the rate of dissociation and the rate of recombination. Reduction of pressure reduces the rate of recombination, whilst leaving the rate of ionization unchanged, and so favours an increased degree of ionization. Saha showed that at a given temperature and pressure the degree of ionization was calculable, given the ionization potential of the atom. The fol lowing table gives the percentage ionization of calcium at 5,000° C. at the pressures mentioned :— The pressure at the base of the chromosphere is probably less than 1 atmosphere, and so in the high-level chromosphere cal cium must be almost completely ionized. The spectrum must therefore be that of the ionized atom, not the neutral atom ; hence the predominance of enhanced lines in the chromospheric spec trum. Similar calculations apply to other elements. The D-lines of sodium, for example, which are due to the neutral atom, are not found in the upper chromosphere, because any sodium there would be completely ionized; the lines of the ionized atom are too far in the ultra-violet to be observable.
The question remains why the chromosphere should extend to such great heights in certain elements. Gravity at the solar sur face is some 27 times as powerful as at the earth's surface, and it is readily calculated that under gravity only the solar atmosphere would have a thickness which would be measured in tens of kilo metres instead of thousands. The explanation is that the atoms are largely supported against gravity by radiation pressure. When an atom absorbs a quantum of light, and thereby undergoes a transition from one stationary state to another, it experiences a blow in the direction in which the light was moving. In the case of an atom near the sun, this blow will be in an outward direction. After a short interval the atom must omit the quantum, suffering thereby a blow from the recoil, but the recoil-blows will be ran dom in direction and will neutralize one another on the average. The net result is that in a succession of absorptions and emissions the atom experiences a series of outward blows. The atom of ion ized calcium in the high-level chromosphere, for example, expe riences some 20,000 such blows per second, and this is just suffi cient to keep it suspended against gravity.
In the absence of a chromosphere, radiation pressure on cal cium atoms at the sun's surface would exceed gravity. Ionized calcium atoms would be expelled from the sun until a screen of atoms was brought into existence sufficiently thick to shield the atoms at the highest level from the direct radiation of the sun to such an extent that radiation pressure was reduced to equality with gravity. This is presumably the mode of formation of the chromosphere ; it is called into existence to redress a dynamical want of balance. The extent to which different kinds of atoms are hoisted in this way by radiation pressure depends on the atomic structure, the position of the lines in the spectrum and the stationary states from which they originate. On these principles it has been found possible to account, generally speaking, for the main features of the chromosphere, and in particular to explain why heavy atoms are sometimes found higher up than the light ones. At high levels in the chromosphere the pressure is very small, probably only some ' o 12 atmosphere, and collisions of atoms with one another are quite infrequent. The equilibrium of an atmosphere under gravity and radiation pressure differs con siderably from that under gravity and a pressure gradient, and to it the name "chromospheric equilibrium" has been applied.
The intensity of H and K radiation to which a high-level atom of calcium is subject is simply the residual intensity in the centre of the dark Fraunhofer H and K lines. Observations of this in tensity combined with the theory of equilibrium under radiation pressure lead to an evaluation of the period (I.8 X Ior seconds) during which a calcium atom remains in an excited state. Chromo spheric theory thus has an important by-product in atomic theory, as the period of excited life of an atom should be an atomic con stant independent of solar conditions. Chromospheric theory has further suggested an explanation of the motions observed in solar prominences, and of the emissions of layers of gases from Novae.
An outstanding problem is the rotation of the chromosphere. The high-level chromosphere rotates round the sun's axis faster than the reversing layer, and moreover does not share the polar retardation experienced by the latter. No satisfactory explanation of this has yet been suggested.
