Uses of Chlorine
USES OF CHLORINE In the days of the Weldon and Deacon processes the principal outlet for chlorine was in the manufacture of bleaching powder (q.v.). Although a very considerable quantity of this is now pro duced, its place has been taken to a great extent by liquid chlo rine itself. Chlorine has an immediate and disastrous effect on all forms of low organic life, and is therefore an ideal disinfectant. The quantity required, for example, to sterilize potable water, or swimming pools, completely is extremely minute and, when the treatment is properly carried out, no taste can be detected. Over 75% of the drinking water of North America is sterilized with chlorine. The death rate from typhoid has dropped from 28 per I00,000 in 1908 to less than five per r oo,000 in 1927, and much of this improvement can be traced to the use of chlorine. This, and the application to sewage, is daily finding more ex tended use. Chlorine in the form of hypochlorite is the potent ingredient of the familiar Carrel-Dakin solution and other types of germicides. It is frequently found that metal surfaces, such as condenser tubes, which are in constant contact with water, de velop considerable growths of algae and similar vegetable matter, which impair the efficiency of the apparatus. These growths can be entirely inhibited by the addition of very small quantities of chlorine to the water, and the efficiency of the plant restored to the normal.

The biggest outlet for chlorine is the pulp and paper trade. As is well-known, modern newsprint and paper of the inferior sorts generally are made from wood which has been shredded and beaten to a pulp. This requires bleaching before it can be used as paper; and to this is due the rise of the chlorine industry in North Amer ica. Bleaching powder (q.v.) was at one time extensively used for this purpose, and is still to some extent ; but in Canada and the United States the change over to chlorine itself is very marked, and the same tendency is showing itself to an increasing degree in Europe.
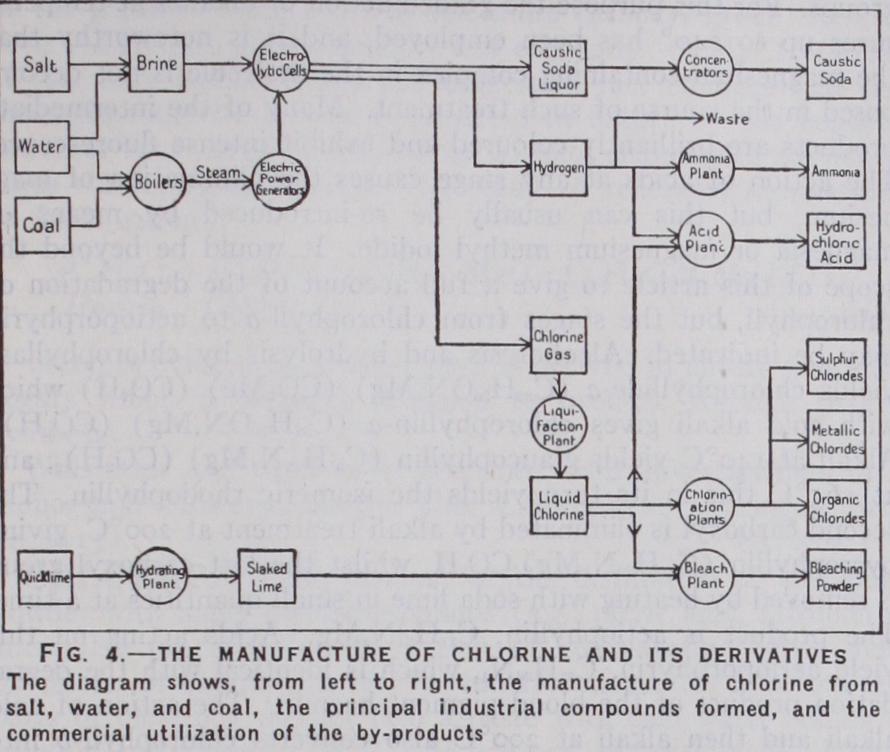
In the manufacture of artificial silk large quantities of chlorine are involved. In the viscose process the source of cellulose is, generally, wood pulp which, as mentioned above, is bleached by chlorine. Caustic soda is also used and the pure product of the mercury cell is much preferred. In the acetate process liquid chlorine and sulphur-chlorine compounds are required. The rise of the artificial silk trade has, therefore, been very beneficial to the industry in general.
Sodium and calcium hypochlorites, prepared by action of chlo rine on caustic soda and lime respectively, are also used as bleach ing agents.
Both liquid chlorine and bleaching powder are used in large amounts in the refining of crude natural oils.
The organic chemical industry provides an important outlet for chlorine in the manufacture of dyestuffs, insecticides, fire-extin guishing liquids, grease-removers, solvents of different kinds, an aesthetics (chloroform), and various intermediate compounds used in comparatively small quantities throughout the chemical industry. Chlorine is widely used in bleaching flour.
In metallurgy chlorine has been used for the extraction of metals from their ores.
Even from the above abbreviated description of the industry, it will readily be seen that chlorine, like so many other raw materials of the modern world, is one of those products which, without being obtrusive, and, in fact, without being known even by name to the vast majority of people, yet has a profound influence on our daily life. Newspapers, clothes, medicines, colours, sanita tion and petrol, for example, are matters of concern to everyone, and all consume chlorine or its derivatives at some stage. In when first discovered by Scheele, it was a chemical curi osity masquerading under the name of "dephlogisticated marine acid air" ; it is now a necessity.
