Fluorescein
FLUORESCEIN, so called from the fact that its dark-red solutions in caustic alkalis, in which it dissolves readily, show a brilliant green fluorescence, especially when they are largely diluted with water. It is a yellow amorphous powder when pre cipitated from water, in which it is insoluble; from alcohol it crystallizes in small dark-red nodules. It was first made in 1876 by A. v. Baeyer by the condensation of phthalic anhydride with resorcinol at C. The two reacting substances are either heated alone or with zinc chloride, and the melt obtained is boiled out with water, washed by dilute alcohol, extracted by means of sodium hydroxide, the solution acidified and the precipitate well washed with water. By repeating this process fluorescein may be obtained in a very pure condition. It is resorcin phthalein, C20H12O,, with the structure shown below.
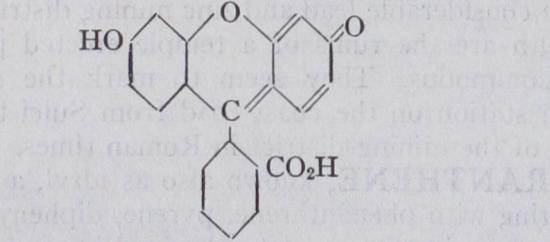
By brominating fluorescein in glacial acetic acid solution, eosin (tetrabromofluorescein) is obtained, which crystallizes from alco hol in yellowish red needles, and dyes silk, wool and mordanted cotton a fine pink colour. The corresponding iodo-compound is known as erythrosin. Other dyestuffs of this series are safrosine or eosin scarlet (dibromodinitrofluorescein) and rose Bengal (tet raiodotetrachlorofluorescein). With zinc dust and caustic soda, fluorescein is reduced to the leuco-compound fluorescein. For the coloured and colourless dimethyl ethers of fluorescein see O. Fischer and E. Hepp . These ethers correspond with the quinonoid and benzenoid (non-quinonoid) modifications of fluorescein. (See DYES, SYNTHETIC.)
