Alkene Addition Reactions
ALKENE ADDITION REACTIONS The general classification of reactions involving alkenes is addition to the carbon-carbon double bond, forming two new single bonds. The term stereoselective describes a reaction which gives a preferred stereoisomeric product in preference to others that are possible from the reaction, and many of the alkene addition reactions are classified as stereoselective. Another term, refers to production of one constitutional isomer during a reaction in preference to others, and many alkene reaction also show regioselectivity. These details of alkene reactions help in establishing reasonable mechanisms for the addition reactions. Three different modes of addition are found which include syn addition - both new bonds form from the same side(face)of the double bond, anti addition - the two new bonds form from opposite faces of the double bond, and addition which is non-stereoselective, showing syn and anti addition products. Inmost cases of syn addition, both new bonds formed simultaneously, without an intermediate species. In cases of anti addition, a bridged, three-membered ring type of intermediate forms by an electrophile binding both carbons of the double bond, which then is opened by a nucleophile from the opposite side. In cases which show both syn and anti addition, a carbocation or free radical intermediate is produced, and free rotation allows the second new single bond to form on either side.
Electrophilic Addition Reactions:
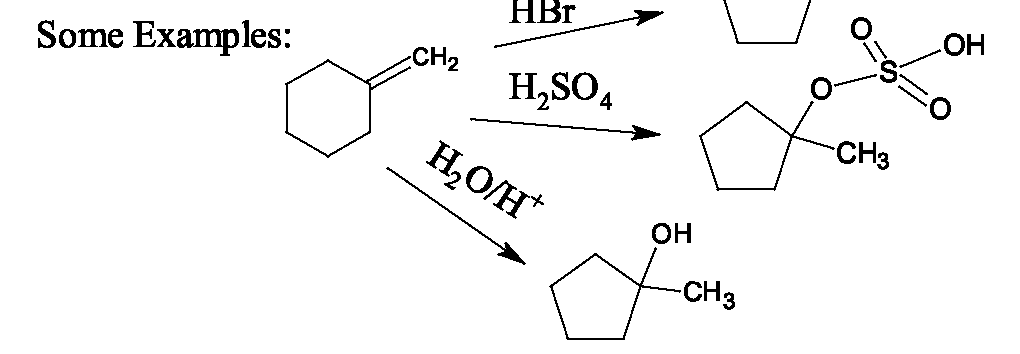
These reactions are so-called because the first step of the mechanism involes an electrophile (electron-seeking species) binding to one (or both) of the carbons of the double bond, producing an intermediate cation. In a second step, a nucleophile attaches and neutralizes the cation's charge. Three examples of electrophilic additions which involve a proton as the electrophile are addition of hydrogen halides, sulfuric acid and water and acid. They all show regioselectivity governed by Markovnikov's Rule which states that the hydrogen of the acid adds to the carbon with more hydrogens. (This places the positive charge which develops on the carbon with more carbons, which is the more stable cation.) The general mechanism for this type of electrophilic addition using E-N as a general reagent which is polarized with partial positive charge on E (the electrophilic end) and partial negative charge on N (the nucleophilic end) is: Because the mechanism involves an intermediate carbocation, rearrangements often occur if the initially formed carbocation can become morre stable due to a tertiary or quaternary carbon that is adjacent to it. For example: Addition of Halogen: Alkenes also react with or by an electrophilic additioon mechanism, with the first halogen adding as a cation and the second one as an anion. Stereochemical studies show that the two halogens undergo anti addition to the double bond, and this provides evidence for a cationic intermediate in which the electrophilic halogen binds to both carbons of the double bond. Then the nucleophilic halogen binds from the opposite side. This mechanism is shown for cyclopentene below.
First, the double bond acts like a nucleophile and displaces from the bromine molecule, resulting in binding of to both carbons. This is more stable than In the second step, the bromide ion acts as a nucleophile on the C-Br bond of the bromonium a carbocation because all atoms have octets in this ion. It must attack from the back-side of the cation. This type of intermediate is generally called a bond that is being broken, so the anti addition halonium ion, and specifically a bromonium ion in this case.
results.
So, the accepted mechanism involves the anti addition, but in many cases it is not evident in the product because of the product's structure. It's very clear on adding to cyclic alkenes, because the halogens will be trans in the product. Rearrangements do not occur during these reactions because of the stability of the halonium ion.
free rotation of the single bond here doesn't really reveal the anti addition. only the stereoiosmers which have the bromines trans to one another are formed.
Draw structures for the products of the reaction of with each of the alkenes below. Illustrate stereochemistry if a preferred stereoisomer is produced.
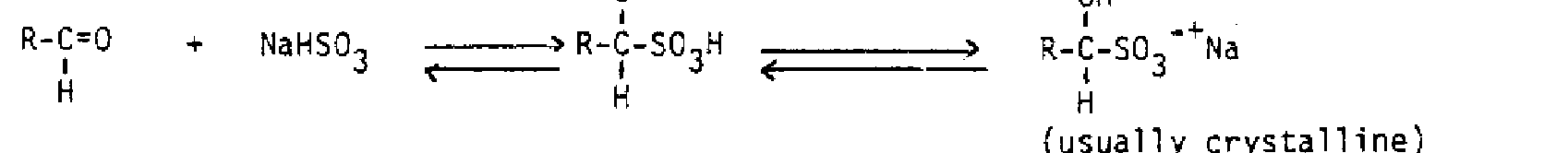
Addition of Halogen in Water:
If halogen reacts with a double bond in aqueous solution, a dihalide does not form. Instead, the water behaves as the nucleophile in the second step and opens up the three-membered ring. Anti addition still results, and if the original alkene was unsymmetrical, the water binds to the carbon with more carbons bound, because it is believed there is more partial positive charge on that carbon. In such a case, the halonium ion itself becomes unsymmetrical, allowing more partial positive charge on the carbon which is more substituted. Addition of bromine in water to methylcyclopentene illustrates all these features.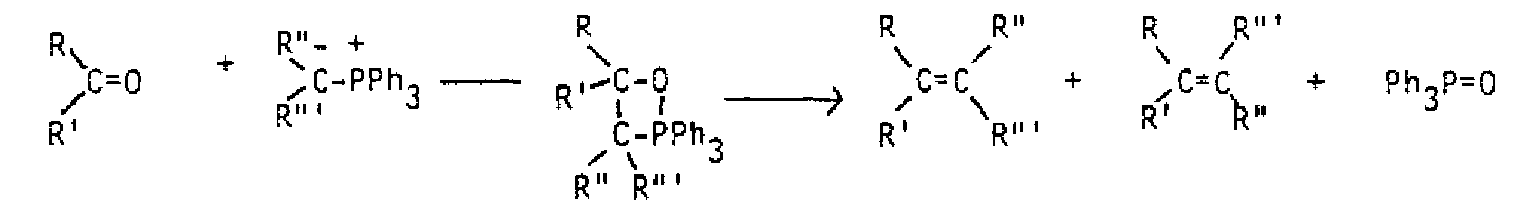
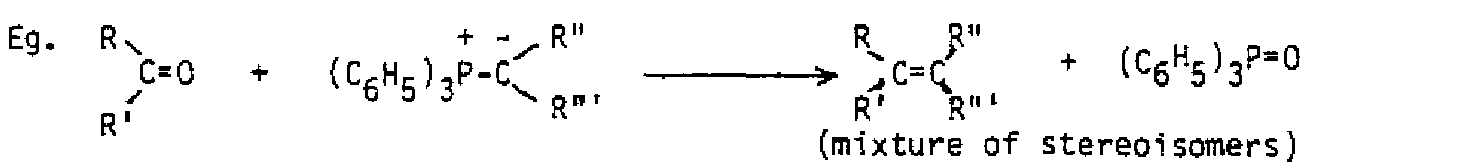
Both stereochemistry (anti) of addition and regiochemistry (nucleophile to more substituted carbon) need to be illustrated.
Draw the structure of the product of each of the following alkenes when treated with chlorine in water.
Anti-Markovnikov Addition of HBr - Free Radical Addition: When HBr reacts with alkenes in the presence of peroxides (which are known to be free radical initiators), the orientation of addition is opposite to Markovnikov's rule. The hydrogen goes to the carbon with more carbons and the bromine goes to the carbon with more hydrogens. This is because the mechanism here involves free radicals, with a bromine atom (radical) adding first, to produce the most stable possible free radical, the more substituted one. This means the bromine binds as a radical to the less substituted carbon. Below is the proposed mechanism. _ The O-O bond of peroxides is weak and easily breaks with light or heat The peroxy radical abstracts H from HBr to yield alcohol and a bromine radical.
The bromine radical adds to the less substituted carbon to leave the odd electron on the more substituted carbon.
Now the alkyl radical abstracts H from HBr, propagating the chain.
The general mechanistic principle of forming the more stable intermediate applies here. Each step is based upon this idea. When the peroxy radical collides with HBr, the reaction takes the path that creates the more stable radical, bromine, rather than hydrogen. This is why the order of addition of H and Br is opposite to that in electrophilic addition. The more electrophilic atom within the HBr molecule is rather than so binds first in that case. This peroxide effect only works with HBr; Addition of peroxides does not change the direction of addition of HCl or HI.
Draw structures for the products of the reaction of each of the above alkenes with HBr/ROOR A second reaction of alkenes that involves free radical addition is free radical In this reaction, a peroxide breaks into peroxy radicals, one of them adds to an alkene to produce and alkyl radical, and the alkyl radical then adds to another alkene to produce a larger alkyl radical. The process repeats until a long-chain polymer is formed. Many substances other than peroxides act as initiators, but the overall result of many alkene molecules (monomers) combining in a specific direction to form a polymer (many parts)is the same in each case. In alkene polymers, the electrons of the double bond are used to join together the alkene units.
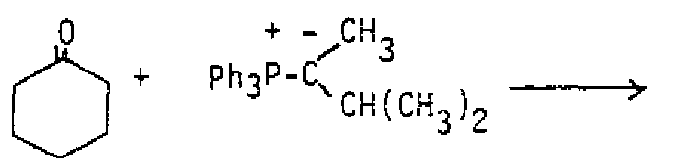
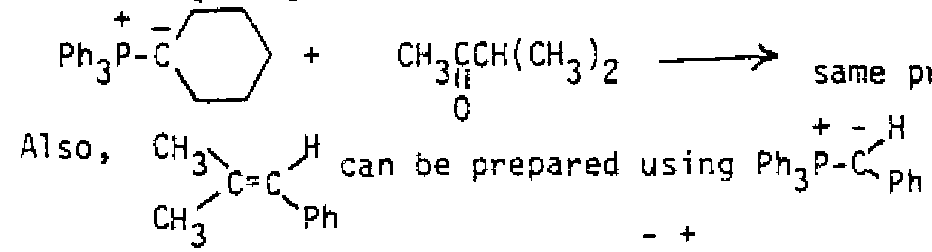
Polymers are too large to show the full structure, so the repeating unit is shown in brackets. This simply turns out the be the alkene structure without the double bond, since those electrons were used to join the units together. Any substituent on one of the alkene carbons repeats at every other carbon of the polymer because of the specific direction of free radical addition. Cationic polymerization is also possible, using an acid to catalyze the process, with similar results. Some examples of well known polymers are shown below.
polyethylene (polymer of ethene): polytetrafluoroethylene(teflon): Syn Addition Reactions: Both new bonds form on the same face of the double bond in this case. Usually both new bonds are forming at the same time. Three reactions show this type of stereochemistry.
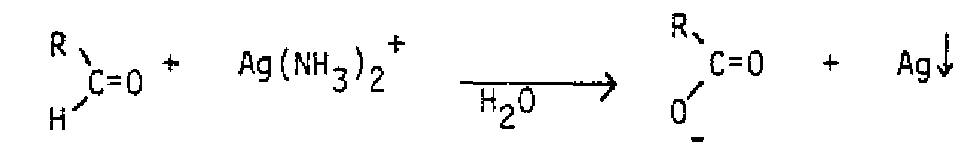
Catalytic Hydrogenation:
Alkenes react with on the surface of a transition metal catalyst, typically Pd, Pt, Ni or Rh, and are converted to alkanes. Because the catalyst is bonded to one face of the alkene, transfer of hydrogen from the catalytic surface takes place on the same face of the alkene.
This case illustrates the syn stereochemistry, producing only the cis 1,2-dimethylcyclopentane and no trans product.
Hydroboration-Oxidation:
This is an involved process, but the net result is syn addition of water across the double bond, but in the anti-Markovnikov direction. The OH ends up on the less substituted carbon. This is a two-step process. First (borane) adds to the double bond, with boron (the more electrophilic part of the reagent), as adding to the less substituted carbon and hydrogen to the more substituted carbon. The actual form of the hydroboration reagent varies. It exists as either diborane, used in diglyme, or borane, complexed with tetrahydrofuran (THF). In a second step, with NaOH is used to oxidize the hydroboration product, leading to replacement of boron, which was on the less substituted carbon, by OH. Some examples are shown below.
This case shows the result of the syn addition. H and OH adding to the same side leads to the OH ending up trans to the Draw structure for the products of each of the alkenes below with a) b)1. 2. Epoxidation: This involves creating a three-membered ring containing oxygen (an epoxide) by treatment of the alkene with a peroxyacid. The proposed mechanism has a cyclic transition state in which electrons reorganize in a single step, so both of the new bonds to the oxygen are formed simultaneously, leading to syn addition. Groups on the alkene that were cis will be cis in the epoxide that forms; trans groups remain trans.
Ozonolysis:
Ozone is a strong electrophile that adds to alkenes to produce an unstable product called an ozonide.The ozonide easily breaks apart in water (hydrolysis - cleavage with water) to give two carbonyl compounds. Depending on the substituents on the double bond, aldehydes, ketones or carboxylic acids are produced. Aldehydes are easily oxidized to carboxylic acids, so often zinc is used during the hydrolysis step to keep the aldehyde from being oxidized to the carboxylic acid. The overall result is that the carbons of the double bond are separated and form new double bonds to oxygen.
Cyclic alkenes will give an open-chain dicarbonyl compound, so this process is useful for creating compounds with functional groups at the opposite ends of a carbon chain.
Often isolation and identification of ozonolysis products can be used to deduce the structure of an unknown alkene. For example, if a isomer was found to give the ozonolysis products shown below, the structure of the alkene must have been cis- or trans-2-hexene. Ozonolysis products: Exercises: If the ozonolysis products of a isomer are the starting alkene? 3 Synthesis These incorporate any of the reactions learned to date. Suggest a sequence of reactions to convert: a. 1-methylcyclopentanol to trans-2-methylcyclopentanol b. 2-bromobutane to 1-bromobutane c. isobutane to 2-methyl-1-propanol d. 1-bromo-1-methylcyclohexane to