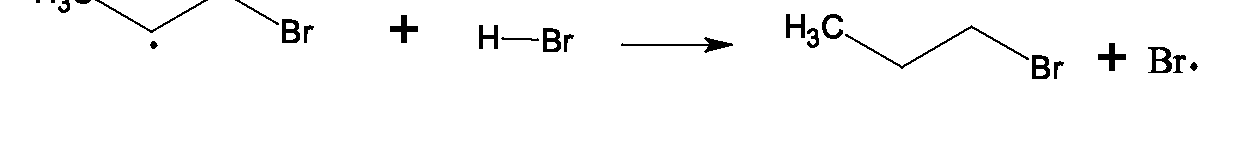Alkyl Halides - Preparation from Alcohols and Alkanes
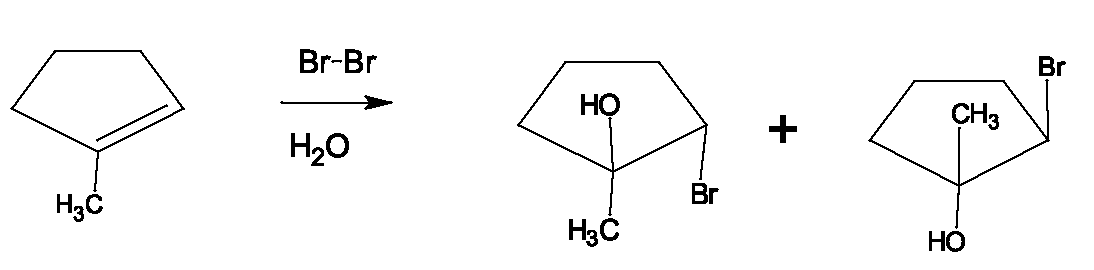
ALKYL HALIDES - PREPARATION FROM ALCOHOLS AND ALKANES From alcohols: Reaction of alcohols with hydrogen halides produces alkyl halidesand water: Since (halide) essentially replaces this reaction is classified as a substitution reaction. Also, since the substition involves electron-rich particles, it is termed nucleophilic substitution. A nucleophile (literally nucleus-lover) is an electron-rich species seeking a nucleus to share its electrons with. The partially positive carbon of the alcohol, or a carbocation formed when water is lost, is classified as electrophililc (electron-loving) because electrons are pulled away from it or removed when a bond breaks. The reaction between a nucleophile and an electrophile can be viewed as a Lewis acid-base reaction, with the nucleophile acting as a Lewis base and the electrophile acting as the Lewis acid.
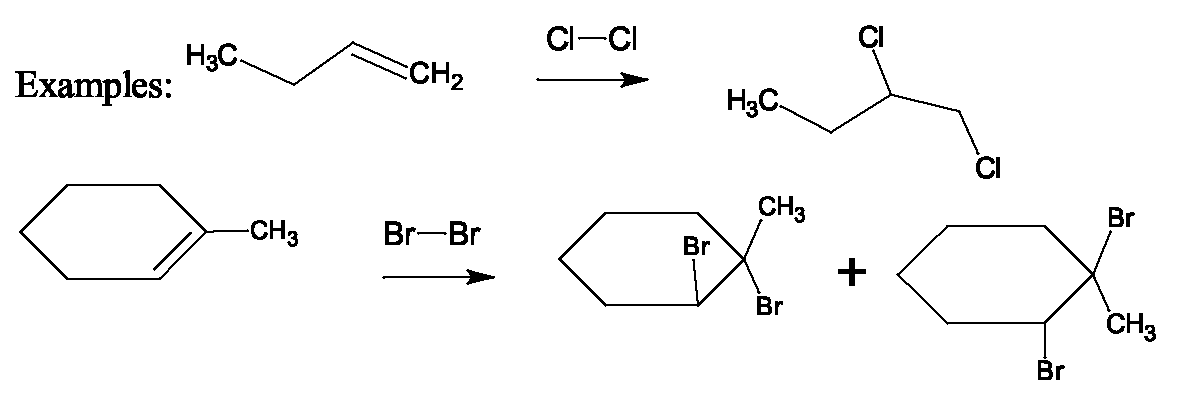
Examples: Draw the products of each of the following reactions: Mechanisms: Two different mechanisms for this reaction operate, depending on the alcohol structure. For secondary and tertiary alcohols, it is believed that a carbocation intermediate is produced. A three-step mechanism is proposed which involves: 1. Protonation of the alcohol, an acid-base reaction, (without a strong acid, the reaction doesn't occur.) (fast - acid-base reaction are very fast processes) This makes the C-O bond cleave more readily because loss of a weak base, is much more favorable than loss of a strong base. In a nucleophilic substutition reaction, the molecule or ion that is replaced is called the leaving group, and as a general rule, this must be a weak base.
2. Loss of from carbon to leave a carbocation.
This can only occur if is a secondary or tertiary carbocation. This is the slow or rate-determining step of the mechanism because it has the highest activation energy. The carbocation is not very stable, since it lacks its octet, and so is difficult to form. Carbocation stability follows the order: Methyl and primary carbocations are too unstable to form. For this reason, methyl and primary alcohols undergo a different mechanism.
3. Combination of with the carbocation, a fast step because it neutralizes the unstable carbocation, returns the carbon to an octet.
The mechenism is designated as substitution, nucleophilic, unimolecular, because the slow step involves only one species, the protonated alcohol, and its rate will be proportional to only that one concentration.
Show all steps in the mechanism for the reaction in problems (a) and (c) above.
For methyl and primary alcohols, the reaction still occurs, but at a slower rate than tertiary or secondary alcohols. Because methyl and primary carbocations are so unstable, the proposed mechanism does not generate these as intermediates.
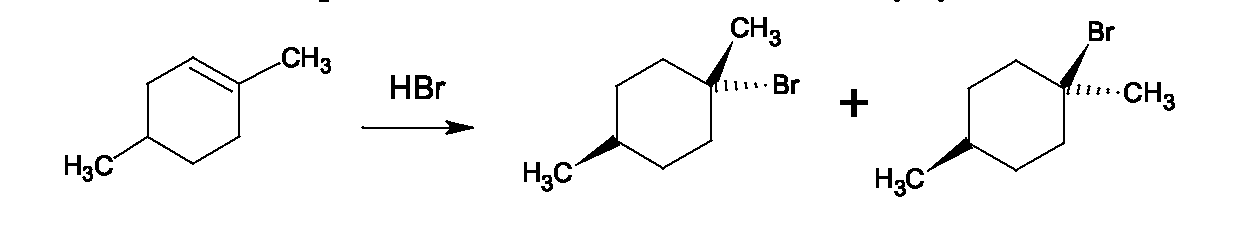
1. Protonation of the alcohol: (This is generally the first step of any reaction between an alcohol and acid.) 2. Now, because the loss of would be too difficult, the nucleophile, helps by displacing the This is easier than loss of and is presumed to occur in methyl and primary alcohols because they are less sterically hindered than tertiary and secondary alcohols. The transition state for the reaction is crowded, since the nucleophile and leaving group are both partially bonded to the carbon. Fewer alkyl groups around the OH-bearing carbon make this transition state possible in these cases. This is the slow step, because it involves bond-breaking (C-O) and bond making (Br-C), so the rate depends on both bromide ion concentration and concentration of the protonated alcohol. It is classified as - substitution, nucleophilic, bimolecular.
Show all steps in the likely mechanism for exercise (b), above.
Other reagents which convert alcohols to alkyl halides are and Decide which type of mechanism should operate for each of the alcohols below when treated with HBr.
Preparation from Alkanes:
Alkanes are non-polar molecules, and the type of bond breaking that is typical of them is termed homolytic, one electron going to each of the atoms of the bond.
(requires much energy - 435 kj/mole) Halogens are also non-polar and can cleave similarly, but require less energy.
When is treated with bromine or chlorine in the presence of heat or light, substitution of hydrogen by halogen occurs, but because homolytic bond cleavage occurs, it is termed free radical substitution, since the intermediates in this case are free radicals (species which contain an odd, unpaired electron).
and are also formed in the reaction. To limit to monosubstitution, excess is used.) Experimental evidence supports the free radical chain mechanism shown below.
Light or heat provides the energy to break the bond.
l The chlorine radical abstracts a hydrogen atom and generates a methyl radical.
Collision of the methyl radical with a chlorine molecule leads to chlorine atome abstraction, and generation of another chlorine radical to continue the chain. Steps 2 and 3 can repeat over and over to produce many product molecules from the initially cleaved chlorine molecule.
Occasionally steps occur in which two radicals combine and cause the end of a chain, and are called chain termination steps. The possible chain termination steps in methane chlorination are: The fact that trace amounts of ethane are produced during chlorination of methane supports the mechanism.
Draw structures for the products of the reactions of chlorine and light with: a. ethane b. cyclohexane c. 2,2-dimethylpropane d. propane e. 2-methylpropane What are the possible structures of larger alkanes which may form as trace products in the chlorination of a. ethane b. 2,2-dimethylpropane c. cyclopentane The mechanism for bromination of methane is essentially the same as the mechanism for chlorination. Bromine radicals, however are more stable than chlorine radicals, and possess less energy. They react more slowly with methane than a chlorine radical does.
Halogenation of Higher Alkanes:
Usually a larger alkane has more than one type of hydrogen for abstraction by halogen. Propane, for example, has 6 primary hydrogens and 2 secondary hydrogens. A careful study of the products of chlorination of propane shows that more 2-chloropropane is formed than 1-chloropropane, although it appears that 1-chloropropane is statistically favored.
This product distribution suggests that there is a lower energy pathway to 2-chloropropane, and it can be shown that abstraction of a hydrogen is easier than abstraction of a hydrogen, because the resulting free radical is more stable. Studies of other alkanes indicate that the relative rates of abstraction of hydrogens by chlorine from alkanes is in the ratio of Usually when an alkane is chlorinated, a mixture of all possible isomers results. Both statistical factors (numbers of each type of hydrogen) and energy factors (stabilities of the possible intermediates) are at work. The relative stability of alkyl radicals is the same as that seen for carbocations: Bromine, on the other hand, is less energetic and selectively abstracts the hydrogen that leads to the most stable possible free radical intermediate. Its relative rate of abstraction of hydrogen from alkanes is also > but in a ratio of In the bromination of an alkane, a purer product is usually obtained, the one that involves the more stable free radical intermediate. Bromination of propane yields 97% 2-bromopropane and only 3% 1-bromopropane. If a hydrogen is present in an alkane, bromination will involve specific removal of that hydrogen over all others, since bromine is so more more reactive towards hydrogens.
Draw structures for the products of the reactions of 2-methylbutane, methylcyclopentane, and 2,3-dimethybutane with a) chlorine and light b) bromine and light Try to predict the major product.