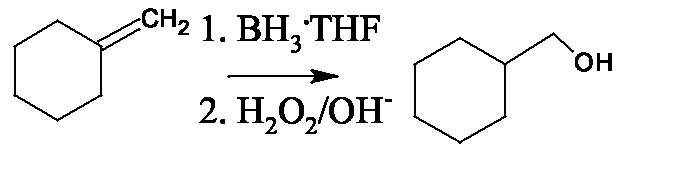Alkynes
ALKYNES Structure: A carbon-carbon triple bond is present. The triply bonded carbons are sp-hybridized. They are named based upon the longest chain containing the carbons of the triple bond, with the suffix -yne replacing -ane of the alkane chain. Number the chain from the end closest to the first carbon of the triple bond. If an OH is present, it gets the lower number. Triple bond gets a lower number than alkyl or halogen.
Acidity of an sp C-H bond:
Alkynes which have the triple bond at the end of the chain are called terminal alkynes. They have hydrogen bonded to the carbon of the triple bond. This type of C-H bond shows raised acidity because the anion left behind has its electrons in an sp hybrid orbital. As a general rule, the more s-character of a hybrid orbital, the closer the orbital is to the nucleus, and the more stable an electron pair in that orbital becomes. This makes that hydrogen more acidic; it has a of around 26, and the base amide ion can remove it.
With hydroxide or alkoxide, the equilibrium is not favorable, because they are weaker bases than the conjugate base of the alkyne. RC=C-H + + ROH weaker acid weaker base stronger base stronger acid Beacuse of the strong basicity of the anion of a terminal alkyne (called an acetylide ion), they cannot be formed or used in solvents which contain OH groups because they would react as a base toward the OH group and deprotonate it. Usually they are formed in liquid ammonia solution because ammonia is a weaker acid than the acetylide anion.
Synthesis of larger alkynes from acetylene:
Once the proton is removed from a terminal alkyne, a nucleophilic anion is produced. It can be used to displace halogen in an reaction from a primary alkyl halide. Secondary and tertiary halides can't be used because they would tend to undergo E2 due to the strong basicity of the acetylide ion. (Remember that hydroxide and alkoxide give major E2 products on and substrates, and the acetylide ion is a stronger base than these) Write the steps needed to prepare 1-pentyne and 3-hexyne from acetylene.
Elimination Reactions used for Alkyne Preparation:
Just like preparation of alkenes, alkynes are prepared by elimination reactions. However, double elimination is necessary to form a triple bond, so dihalides are the starting coupounds. They may be geminal dihalides (both halogens on the same carbon) or vicinal dihalides (halogens on adjacent carbons) which are usually prepared from alkenes. The stronger base sodium amide is used, because the removal of the second hydrogen halide is more difficult than the first one. Two equivalents of base are required for an internal alkyne but three equivalents for a terminal alkyne because of the acidity of the sp C-H bond that forms. The base will selectively remove this proton as the terminal alkyne begins to form, rather than dehydrohalogenating the vinyl halide that is produced during the reaction. The end product from the terminal alkyne is the sodium salt of the alkyne. It can be neutralized by water treatment. The combination of alkene halogenation, followed by double dehydrohalogenation is a standard method of converting alkenes to alkynes.
Alkyne Reactions:
Just like alkenes, alkynes undergo addition reactions. They can add up to two moles of reagent per mole of alkyne. In some cases special conditions are needed to control addition to only one mole of reagent.
Reduction to alkanes or alkenes:
Catalytic hydrogenation of alkynes produces first alkenes, which can be further hydrogenated to alkanes. Because alkenes hydrogenate faster than alkyn es, special catalysts are used if the alkene is the desired product. RC=CR H cis RCH=CHR In this reaction, the alkene can't be isolated because as soon as some of it forms, the hydrogenation occurs faster on it rather than the remaining unreacted alkyne. Many special catalysts are available to stop hydrogenation at the alkenestage, and a popular one is the Lindlar catalyst, which contains PbOAc (lead acetate) and quinoline in addition to the Pd, commonly called Lindlar Pd. This provides a very useful preparation of purely cis alkenes from internal alkynes. Another method is available to prepare purely trans alkenes from internal alkynes, in which a group I metal, usually Na or Li is used with The mechanism involves electron transfer from the metal followed by proton removal from ammonia, with radical anion-type intermediates. Along this pathway, the radical anion with the trans alkyl groups is more stable, and leads to stereoselective formation of the trans alkene.
Addition of Hydrogen Halides:
Hydrogen halides add with Markovnikov orientation to the triple bond. Addition of one mole of HX gives a vinyl halide, and addition of two moles gives a geminal dihalide.
The addition of the first HX is thought to occur through a transition state in which both and bind simultaneously from different HX molecules, rather than through an unstable vinyl carbocation. The addition of the second HX is always in the same direction as the first one and proceeds through a resonance-stabilized carbocation formed by protonation at the carbon adjacent to the halogen, placing the + charge on the X-bearing carbon. Its lone pair will stabilize the cation by resonance. The second halogen ultimately binds there.
Addition of Halogen:
One or two equivalents of or can be added to an alkyne, producing either a vinyl dihalide or a tetrahalide. The anti addition is evident when one equivalent is added to an alkyne.
Addition of Water (Hydration):
Water can add to an alkyne using acid catalysis along with a mercury (II) salt, usually Markovnikov's rule is followed in the case of terminal alkynes. The product of water addition, however, is never isolated because it quickly rearranges to a carbonyl compound, a ketone in all cases, except when acetylene is the alkyne.
unstable enol rearranges to the ketone. C=O more stable than C=C Aldehydes can be produced if anti-Markovnikov addition of water is done, using the hydroboration-osidation process.
(It's not really necessary to draw the structure of the enol intermediate product that is formed, since it is never isolated.) Ozonolysis of Alkynes: Alkynes undergo ozonolysis, just as alkenes do, and because two equivalents of ozone can add, the products are carboxylic acids. Terminal alkynes give carbonic acid from the terminal carbon. Zn does not need to be used in the hydrolysis step, because aldehydes can't be produced. It's used in alkene ozonolysis to prevent aldehyde oxidation.
Draw structures for the products of the reaction of 1-pentyne with: Draw structures for the products of 2-hexyne with reagents a-f, h above.
Synthetic Applications:
From a simple alkyne, many larger compounds with different functional groups can be prepared using the appropriate reagents. For example, acetylene can be converted to all the reagents needed to prepare a compound such as 1-butyne. First the acetylene must be converted to a haloethane. Then this can be treated with a sodium acetylide to give the 1-butyne.
Show all synthetic steps and products along the pathway for each of the multi-step conversions below.
a. trans-2-pentene to cis-2-pentene b. propene to 2-hexyne c. 1-butyne to 3-heptanone d. acetylene to butanal (the 4-carbon aldehyde) e. acetylene to 2-chloro-1-pentene f. 1-butene to 2,2-dichlorobutane g. acetylene to 1-butanol h. acetylene to ethyl propyl ether