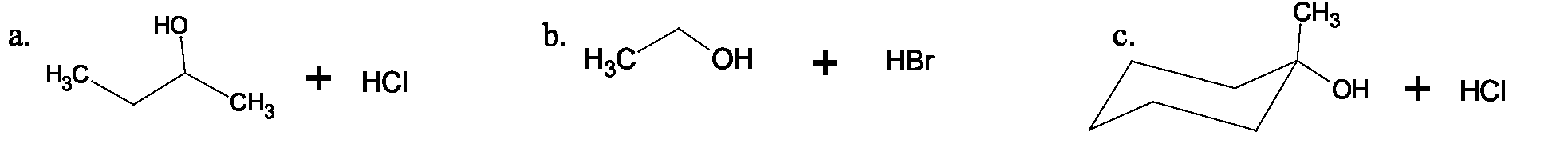Conjugation and Resonance
CONJUGATION AND RESONANCE A conjugated system has an array of two or more hybridized atoms. Since each atom has hybridization, p orbitals are available on each of the atoms of of the system, and overlap of electrons in these orbitals is possible. In allylic intermediates, three p orbitals exist on adjacent atoms, with one pair of p orbitals corresponding to a pi bond. The third p orbital may contain an odd electron, as in an allylic radical, contain an electron pair, as in an allylic anion, or be empty, as in an allylic cation.
In each of these intermediates, the adjacent pi bond allows electron delocalization (resonance) of the intermediate's odd electron, poritive or negative charge, giving it additional stability. Each of the above valence bond structures has an equivalent resonance contributor in thich the odd electron, positive or negative charge is accommodated by the other end of the allylic intermediate.
Neither valence bond structure adequately describes these allyl intermediates, and the third structure is a more accurate depiction of the electron distribution of the radical, cation or anion.
Orbital pictures of these intermediates show an array of three p orbitals on adjacent atoms, so that no localized pi bond is present, and electron flow among all three orbitals can occur. In these simple (unsubstituted) allylic cases, both contributors are of equal stability, so contribute equally to the overall resonance hybrid. When alkyl groups are present at one of the ends of the allylic system, they will increase the stability of the allylic cation or radical which has its odd electron or positive charge on that carbon.
The left hand contributor is more stable (secondary vs. primary allylic) The tertiary allylic contributor is more stable than the primary allylic one and contributes more to the overall resonance hybrid.
Relative Stability of the Allyl Radical:
Bond dissociation energies for C-H bonds indicate the allyl radical to be more stable than even tertiary radicals. Allylic C-H bonds are easier to break homolytically than tertiary C-H bonds, presumably because the resulting radical is more stable.
Radical Stability: allylic > tertiary > secondary > primary > methyl > vinyl Carbocation Stability: tertiary > allyl, secondary > primary > methyl > vinyl Solvolysis rates are used to determine relative carbocation stability; the simple allyl cation is nearly as stable as a tertiary cation, while a secondary allylic cation is more stable that a tertiary cation. One piece of evidence for allylic cations during solvolysis is the allylic rearrangement product often found.
There is more partial plus charge on the tertiary carbon, so this leads to the major product.
Draw structures for all products formed in each of the reactions shown below. Draw the structure of the intermediate in each case and include all resonance contributors.
Allylic Free Radical Halogenation: Under free radical conditions, alkenes which have allylic hydrogens undergo allylic substitution by halogen rather than addition to the double bond. Chlorination or bromination at high temperatures or with UV light leads to formation of allylic chlorides or bromides, rather than vicinal dibromides. The substitution is favored more if a low molecular halogen concentration is used.
A reagent specifically chosen for allylic (and benzylic) halogenation of N-bromosuccinimide (NBS), with heat or light. This compound supplies a steady low concentration of molecular halogen, which reacts with alkenes by a chain mechanism involving abstraction of an allylic hydrogen. The allylic halogenation reaction is most useful when only one product is formed. The alkene must be symmetrical, and so must the allylic radical produced on hydrogen abstraction. In the case below, the alkene is symmetrical, but the allylic radical is not, so two products form.
Draw all products when each of the alkenes below is treated with bromine and light. (only one gives a single product.)