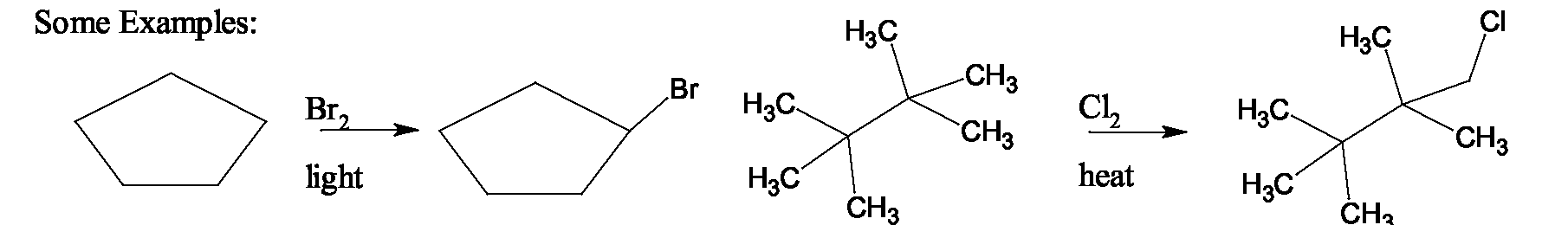
Elimination Reactions of Alkyl Halides
ELIMINATION REACTIONS OF ALKYL HALIDES Dehydrohalogenation of Alkyl Halides: Here, loss of hydrogen halide from an organic compound takes place. Since hydrogen halides are strong acids, strong bases are typically used to bring about this elimination reaction. Examples of the bases used are KOH in ethanol, in ethanol of in t-butyl alcohol.
Compounds like and are alkoxides, the conjugate bases of alcohols, and are used in their corresponding alcohol as the solvent. In the presence of a strong base, all alkyl halides undergo a bimolecular elimination (E2) mechanism. In a single step, the base abstracts the 0 proton, the double bond forms, and the halide leaves with its electrons, as shown below. Rate depends on both alkyl halide concentration and base concentration.
The Greek letters, a and 0 refer to the position of the hydrogen relative to the leaving group. a is on transition state - RO-H bond the carbon bound to the leaving group and 0 is on forming, C-Br bond breaking the adjacent carbon. and C=C bond forming.
The Zaitsev rule states that the more stable alkene is the major product when isomeric products are possible and it applies in most situations when there is more than one direction of elimination. The more stable alkene is the more substituted one, with the most alkyl groups bonded to the carbons of the double bonds. Since no carbocation intermediate is produced, rearrangements do not occur in this reaction. Draw the structures of the products of E2 elimination of each of the following alkyl halides using in and choose the major product if any mixtures are produced.
For elimination on alkyl halides, in is preferred over other bases because halides have a tendency to undergo substitution by the base instead of elimination, since they are less sterically hindered than or alkyl halides. Using makes the base sterically hindered, to cut down on the side reaction of substitution. For example, if 1-chloropropane is treated with in , the
products are 91% only 9% (E2) Use of leads to formation of propene as the major product (over 80%) Suggest the reagent to use for each of the conversions below.
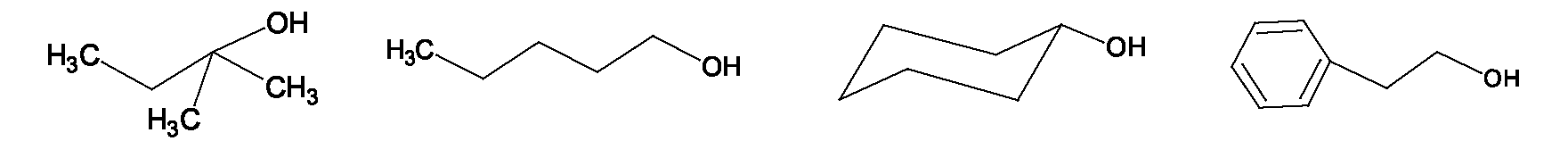
A second mechanism for dehydrohalogenations exists, known as E1, but only occurs in the absence of a strong base. It is not the desired method for preparation of an alkene from an alkyl halide. It's an E1 mechanism, with the alkyl halide ionizing in a slow step to a carbocation and a halide ion when heated in a solvent. Then in a second step, the solvent or halide ion acts as a base and removes the proton from the adjacent carbon. Steps in the E1 elimination for t-butylchloride on heating in methanol are shown below. Rearrangements are possible due to the cation intermediate, so it is not really used as a method of preparing alkenes. It is a side reaction of the reaction.
Suggest an alkyl halide that could be used to produce each of the alkenes below as a major reaction product.
Multistep Synthesis:
Often it takes more than one reaction to convert one functional group to a different one. For example, there is no way to efficiently convert an alkane to a specific alkene by laboratory methods. To make such a transformation, though, one could convert the alkane to an alkyl halide, using free radical halogenation, and then dehydrohalogenate the alkyl halide to the alkene.
Provide the reagents and synthetic steps needed for each of the two-step conversions below.
There is one more detail concerning the E2 transition state for alkyl halides. The proton lost and the halide lost should adopt an anti, periplanar relationship. Sometimes the inability to adopt this relationship leads to a product that doesn't obey the Zaitsev rule. See the example below.
ring flip to allow anti, periplanar relationship