Nucleophilic Addition to Aldehydes and Ketones
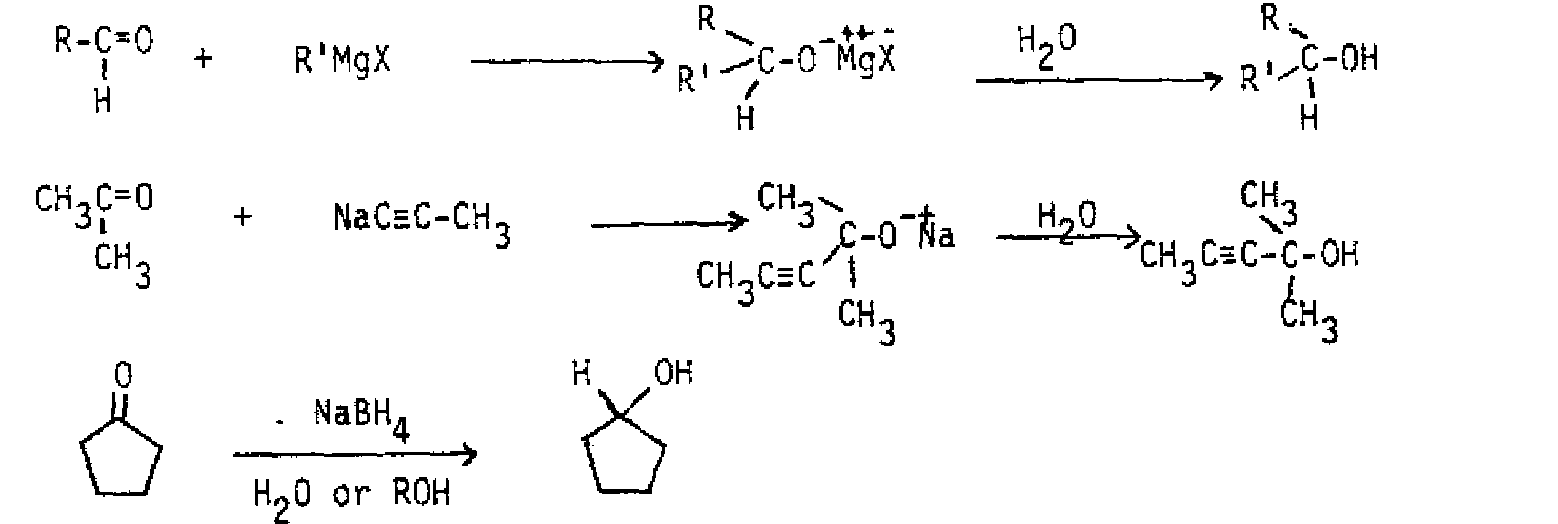
NUCLEOPHILIC ADDITION TO ALDEHYDES AND KETONES A major class of reactions of aldehydes and ketones involves nucleophilic addition to the carbonyl group. Because of the polarization of the carbonyl group, with partial positive charge on carbon, nucleophiles attack the carbon of the carbonyl group, and an electrophile. most commonly a proton or a metal cation, attaches to oxygen.
With a strongnucleophile, the reaction generally involves the nucleophile attacking carbon, displacing the pi electrons to oxygen. A proton or a metal cation then binds to the oxygen.
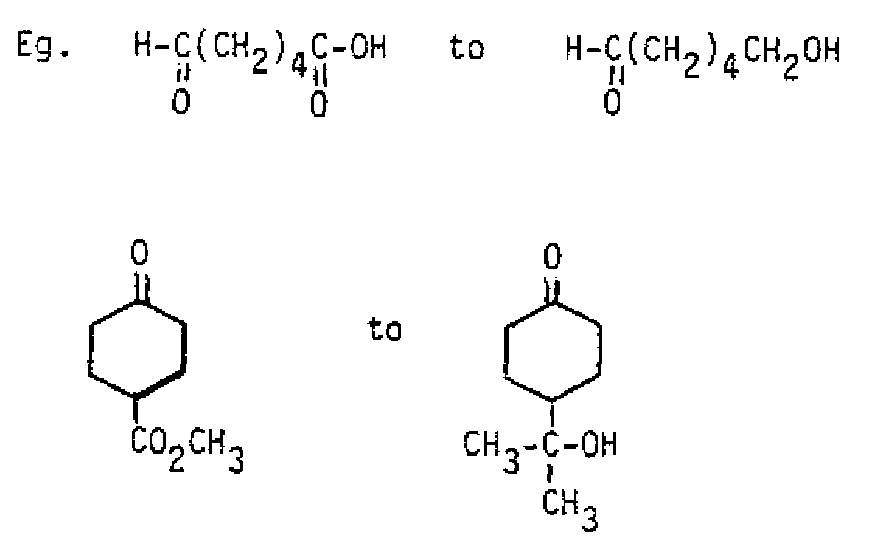
Some examples of reagents of this type include Grignard reagents, alkyllithiums, sodium alkynides, and The reduction of ketones with also yields the corresponding alcohol, but must be done in two steps, since it cannot be used in alcohol or water like A second hydrolysis step using or dilute acid follows the treatment.
Under acidic conditions, the order of events in the nucleophilic addition reaction is reversed. The acid protonates the oxygen of the carbonyl group first, rendering the carbon more positive. Then the nucleophile attacks. This is the typical route with weaker nucleophiles, such as water, alcohols and phenols. These nucleophiles will all add to the carbonyl group without added acid, but the addition of acid catalyzes the reaction.
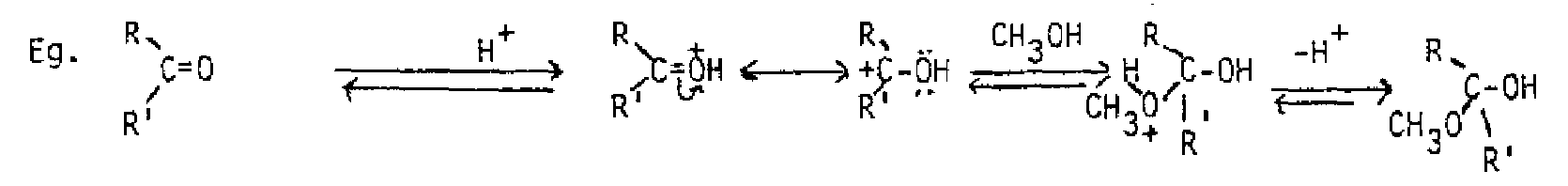
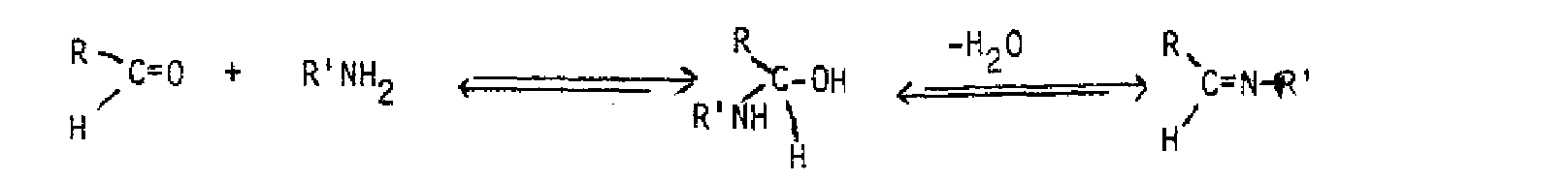
Many of the additions to carbonyl groups are reversible, with the equilibrium favoring the carbonyl compound, so that not all addition products are able to be isolated. In many cases, the initial addition product undergoes a subsequent elimination reaction by loss of water. This is generally the result when ammonia, amines and derivatives of ammonia are used as nucleophiles; very often the product contains a C=N bond in place of the original C=0.
Addition of oxygen nucleophiles:
Both water and alcohols add to the carbonyl group to produce addition products which are usually unstable, and not isolable.
Addition of water products a hydrate, or gem-diol.
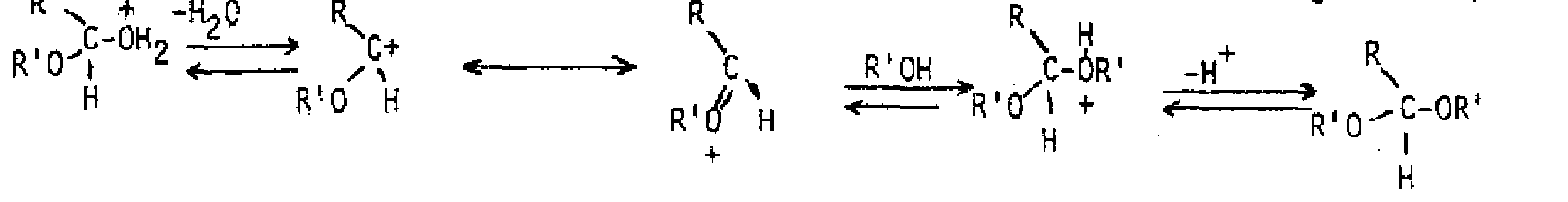
Acid or base can speed up the reaction, but does not affect the position of the equilibrium. Most of the time, the gem-dic can't be isolated from an aqueous solution Addition of alcohol produces a hemiacetal from an aldehyde or a nemiketal from a ketone, If a hemiacetal or hemiketal is treated with acid and another equivalent of alcohol, an acetal or ketal is formed. The overall process is an equilibrium one also, but excess alcohol favors formation of the acetal or ketal. Excess water and acid will convert an acetal or ketal back to the starting aldehyde or ketone. These compounds are actually a special class of ether, so are inert to bases and strong nucleophiles. For this reason, they are often used as protecting groups for the aldehyde or ketone while another part of the molecule is modified.
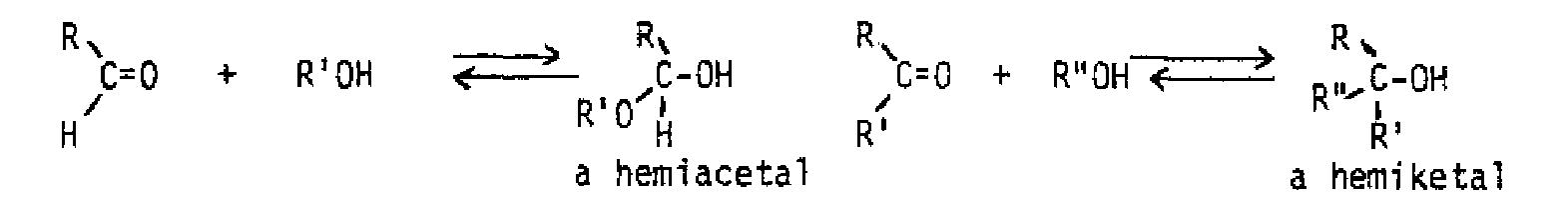
General Reaction: Thepurpose of the acid is to protc-, the hydroxyl so it leaves as water. An especially stable cation with resonance over oxygen is produced. The second alcohol molecule binds then deprotonates to give the produL If a vicinal diol is used, a cyclic acetal or ketal containing a five membered ring is produced. Ethylene glycol is commonly used for this purpose.
Application of acetal and ketal use as protecting groups: The acetal or ketal would be resistant to hydride reducing agents and organometallics. If an aldehyde or ketone is present along with an ester or acid functional group, the ester or acid could be selectively reduced by if the aldehyde or ketone was first converted to the ketal or acetal.
1. (makes the acetal) 2. (reduces the acid) 3. (hydrolyzes acid salt and acetal) 1. (makes ketal) 2. CH 2 equiv. (adds to ester) + 3. (hydrolyzes alkoxide and ketal) Thioacetals and thioketals: The are the sulfur analogues of the acetals and ketals and are prepared by a corresponding route using thiols (RSH) or dithiols in place of the alcohols. Their important use in synthesis stems from their ability to be reduced using Raney nickel to the corresponding hydrocarbon. This is the same result accomplished with the Clemmensen reduction or the Wolff-Kishner reduction, but the conditions are relatively neutral.
Addition of ammonia, amines and derivatives of ammonia:
of these compounds is typically followed by elimination of water to produce a carbon-nitrogen double bond. Whenever the nitrogen has two hydrogens bound to it, the nitrogen loses a proton while the hydroxyi is lost, after the initial addition reaction.
+ R,C-OH R2C=NR 2 NHR (an imine)Nhr(an imine) When a secondary amine undergoes the reaction, the initial addition product has no proton on nitrogen, so the elimination of water is to produce a carbon-carbon double bond, an enamine.

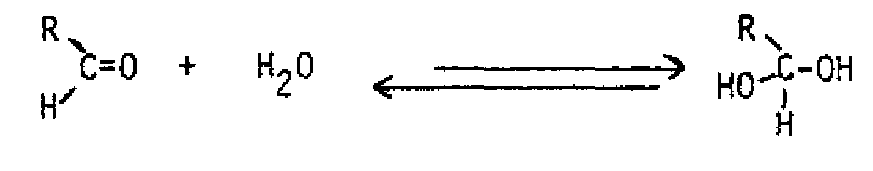
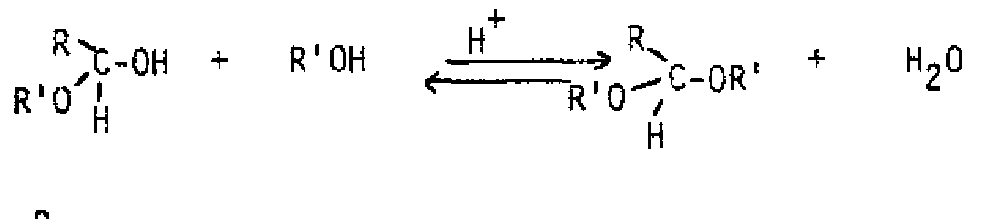
Other derivatives of ammonia farm similar addition-elimination products with aldehydes and ketones and are used chiefly for characterization of aldehydes and ketones because the products are usually crystalline solids with well-known melting points.
If hydroxylamine is used, the product is an oxime.
If hydrazine is used, the product is a hydrozone: R2C=0 + These are intermediates in the Wolff-Kishner reduction. (hydrazone) phenylhydrazine and 2,4-dinitophenylhydrazine form the phenylhydrazone and the 2,4-dinitraphenylhydrazone. These are often crystalline and their melting points are tabulated in reference books.
Another useful ammonia derivative for characterization purposes is semicarbazide, which forms semicarbazones on treatment with aldehydes and ketones.
(notice that the which is not bound to the carbonyl is the one that reacts; it is far more nucleophilic because its lone pair is not delocalized over the carbonyl oxygen.) Cyanohydrin formation: Addition of HCEN to the carbonyl group leads to formation of a cyanohydrin, a compound bearing a hydroxyl and a cyano group on the same carbon. Added cyanide ion catalyzes the reaction, because HCaN is a weak acid, so can't effectively protonate the carbonyl. Cyanide, being a good nucleophile, attacks the carbonyl, and the alkoxide ion then deprotonates HC=N to form the product.
Conversion of thecyaoogroup to a carboxylic acid is accomplished with acid or base and water, so that cyanohydrins are a route to ~-hydroxy acids. Reduction of the cyano group to a primary amine is also possible, using so cyanohydrins are intermediates in the preparation of B-aminoalcohols.
addition: Aldehydes and ketones yield a bisulfite addition product, if the carbonyl is not too sterically hindered. Most aldehydes and methyl ketones give reasonable yields of bisulfite addition product. This is also an equilibrium reaction, and acid or base will cause loss of bisulfite.
The major use of this adduct is to precipitate aldehydes and methyl ketones out of mixtures of other compounds.
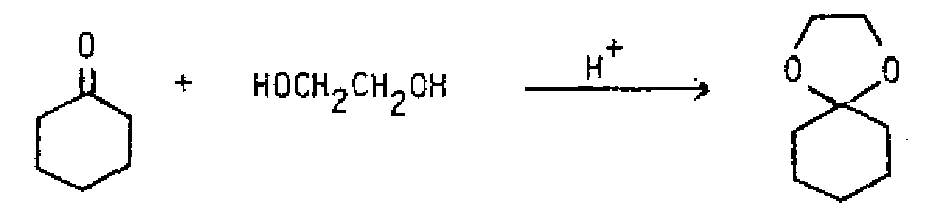
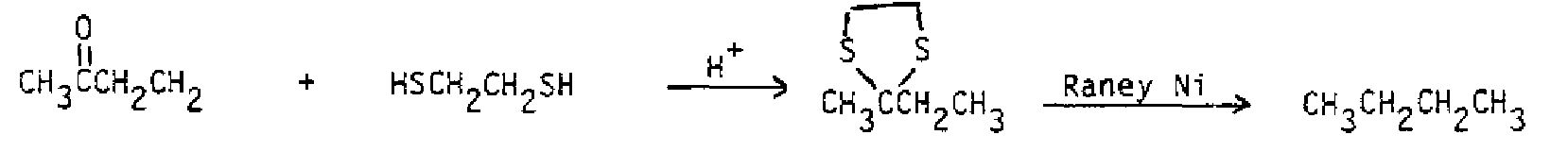
The Wittig reaction: Preparation of alkenes from carbonyl compounds. The net result of this reaction is replacement of the carbon-oxygen double bond by a carbon-carbon double bond. It involves treatment of the carbonyl compound with a phosphorus ylide.
As in other nucleophilic addition reactions, the negative end of the reagent binds to carbon and the positive end binds to oxygen. An unstable four-membered cyclic intermediate called the oxaphosphatane is produced, which collapses to triphenylphosphine oxide and the alkene.
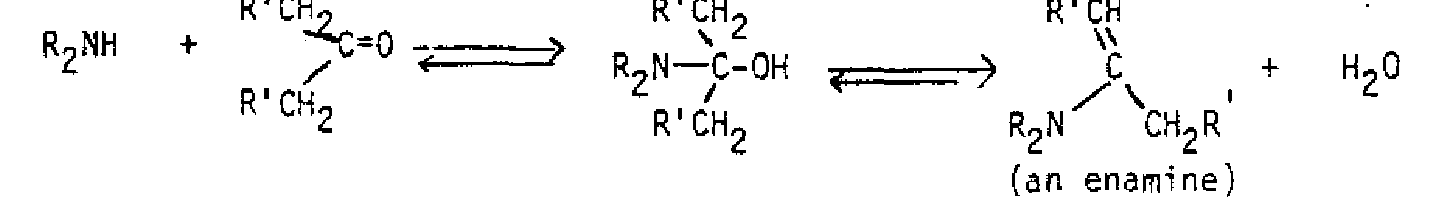
(Ph is an abbreviation for the phenyl group) Preparation of the ylide: The ylide is prepared by treatment of an alkyl halide with triphenylphosphine. A nucleophilic substitution reaction occurs and a phosphonium salt is produced.
Treatment of the phosphonium salt with a strong base, often an alkyllithium, deprotonates carbon, producing the ylide. Only primary and secondary alkyl halides can be used, since a proton must be present for abstraction by base.
Some examples: Often two combinations of Wittig reagent and carbonyl compound can produce the same alkene. The above compound could also be prepared using 3-methyl-2-butanone and the Wittig reagent prepared from a cyclohexyl halide.
roduct as above.
(prepared from a benzyl halide) and acetone The alternate combination, and benzaldehyde, would also work, but the preparation of the Wittig in this case is from a secondary halide, which generally is lower yielding than that from the primary halide.
Oxidation: Aldehydes, but not ketones, are readily oxidized by both strong and mild oxidizing agents. Their ease of oxidation allows them to be distinguished from ketones by treatment with silver nitrate and aqueous ammonia, a reagent known as Tollens reagent.
This reaction is the basis for a test-tube test known as the Tollens or silver mirror test. It is for aldehydes but not ketones.
The product of oxidation of aldehydes by the typical oxidizing agents or KMn04 a is the carboxylic acid.