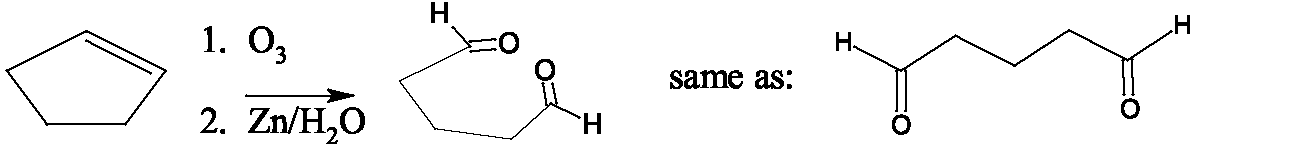Reactions of Alcohols
REACTIONS OF ALCOHOLS A review reaction is acid-catalyzed dehydration to produce alkenes. This is often by an E1 mechanism, so rearrangements are common, and mixtures of alkenes are often formed. The Saytzeff orientation leads to predominantly the most stable alkene possible.
Oxidation reactions: Primary alcohols are oxidized to aldehydes by pyridinium chlorochromate (PCC) in CH2Cl2, (produced by combining HCl and pyridine); secondary alcohols are oxidized to ketones by a variety of Cr(VI) reagents, most commonly with (producing Another reagent that oxidizes secondary alcohols is When primary alcohols are treated with these reagents they are converted to carboxylic acids due to the easy oxidation of the first-formed aldehyde. Tertiary alcohols don’t undergo oxidation readily.
Conversion to tosylates: Replacing the alcohol proton by the tosyl group gives an alcohol the same properties as an alkyl halide. The usual reagent is tosyl chloride in pyridine. The tosylate anion is good leaving group, so substitution and elimination reactions can take place without the use of acid typically used for the simple alcohol. Rearrangements are avoided by use of the tosylate, and stereochemistry of a reaction can be more closely followed. All the nucleophiles used to promote substitution on alkyl halides can be used on the tosylate. Since the reaction is no rearrangements occur, and stereochemical inversion of a chiral carbon takes place.
Reaction with hydrogen halides: Here substitution occurs on the protonated alcohol, using HCl or HBr. Since HCl is the weakest of the hydrogen halides, is often an added catalyst to prepare the alkyl chloride. The relative reactivity with respect to alkyl group is tertiary>secondary>primary>methyl, and generally mechanisms operate for primary alcohols and methanol, while mechanisms (which can have rearrangement) operate for secondary and tertiary alcohols. Several limitations on these reactions include poor yields, competing elimination reactions, rearrangements and difficulty in alkyl iodide preparation. Reaction with phosphorus halides: and (which produces all convert primary and secondary alcohols to the corresponding halide in good yields. A two step reaction in which the second step is by halide ion leads to
fewer rearrangements and often inversion of configuration of a chiral alcohol. Thionyl chloride also is used to make alkyl chlorides and often leads to retention of configuration.
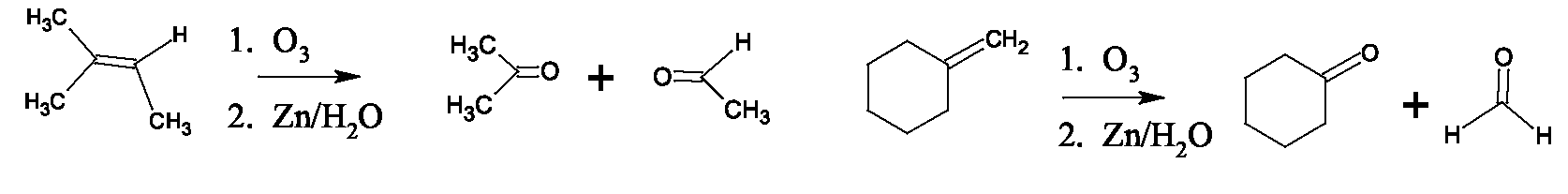
Ether formation: dehydration between two identical primary alcohol molecules using acid catalysis leads to ether production. Temperature control is critical, to prevent the usual intramolecular dehydration to an alkene, and temperatures around 140 favor ether formation while higher temperatures favor alkene formation. This method is only useful for symmetrical ethers. Cyclic ethers are easily produced from 1,4 or 1,5-diols. Unsymmetrical ethers are produced by treatment of primary alkyl halides with alkoxides, an reaction known as the Williamson synthesis. (secondary and tertiary halides do not give good yields due to E2 competition.) The pinacol rearrangement is an acid-catalyzed dehydration of a vicinal diol (glycol) which, accompanied by rearrangement, produces a ketone. Another special reaction of glycols is periodic acid cleavage, which breaks the carbon carbon bond of the glycol and oxidizes the alcohols to aldehydes or ketones. This reaction produces the same products as ozonolysis of the corresponding alkene. Ester production: Carboxylic acids and alcohols (ROH) react in the presence of an acid catalyst to produce an ester This is another example of an dehydration, with the carboxylic acid OH and the alcohol proton forming An alternate method of producing an ester uses an acyl chloride (similar to tosyl chloride) with the alcohol. Ester formation with inorganic acids such as sulfuric, phosphoric and nitric acid is also possible. Total synthesis of complex molecules from simple alcohols can be accomplished by combinations of oxidations and Grignard reactions. Any alcohol can be converted to a Grignard reagent in two steps – conversion to the halide followed by treatment with Mg in ether. Oxidation of an alcohol produced by a Grignard reaction leads to a carbonyl compound which can be again treated with a Grignard reagent.