X-Rays and Crystal Structure
atom, electrons, ions, ion, shell, atoms, shells, stable, outer and fig
To understand the essence of this theory of crystal chemistry, we must start not from the crystal but from the atom. The most convenient form in which to represent the atom for this purpose is by Schrodinger's wave mechanics. Here each atom is pictured as a nucleus of positive electricity, surrounded by shells of diffuse negative electricity, growing denser towards the centre. Each shell corresponds to a set of Bohr orbits with the same chief quantum number in the classical quantum mechanics. These elec tron shells serve in the first place to give the atom a finite size (see fig. 12). This does not mean that the atom is a rigid sphere of definite radius. The wave me chanics atom must be considered as having a definite degree of compressibility and deformabil ity. The chemical and crystal lographic properties of an atom depend partly on the size but more on the character of its outer shell of electrons.
Certain arrangements of elec trons (2, 8, 18, 32 for chief quan tum numbers 1, 3, 4) have their inner quantum numbers so balanced that they are in a condition of minimum energy and so are physically stable and without external electric fields. These arrangements occur in the inner shells of most elements (but not in the iron, palladium, platinum or rare earth groups) but only the elements of the inert gas group have stable outer shells with 8 elec trons. The other elements tend to fall into two groups. Those with one, two, or rarely up to four or five electrons more than the next lower stable grouping are the metals. They all tend to ionize, that is, to lose these spare electrons and assume a more stable con figuration, acquiring a positive charge as a result ; thus a sodium atom with eleven electrons loses one readily to become a singly charged sodium ion Na+, with ten electrons in the configuration of the rare gas neon. The non-metallic type of atom is, on the other hand, one, two or three electrons short of the number required to form the next stable configuration and tends to take up extra elec trons to complete the shell, acquiring a negative charge in the process.
Isolated atoms and ions only exist at temperatures and pres sures such as we find in stars and vacuum tubes. Under normal conditions they are always found combined. We know four such types of combination. These are called the heteropolar or ionic, the homopolar, the molecular and the metallic.
Ionic Combination.
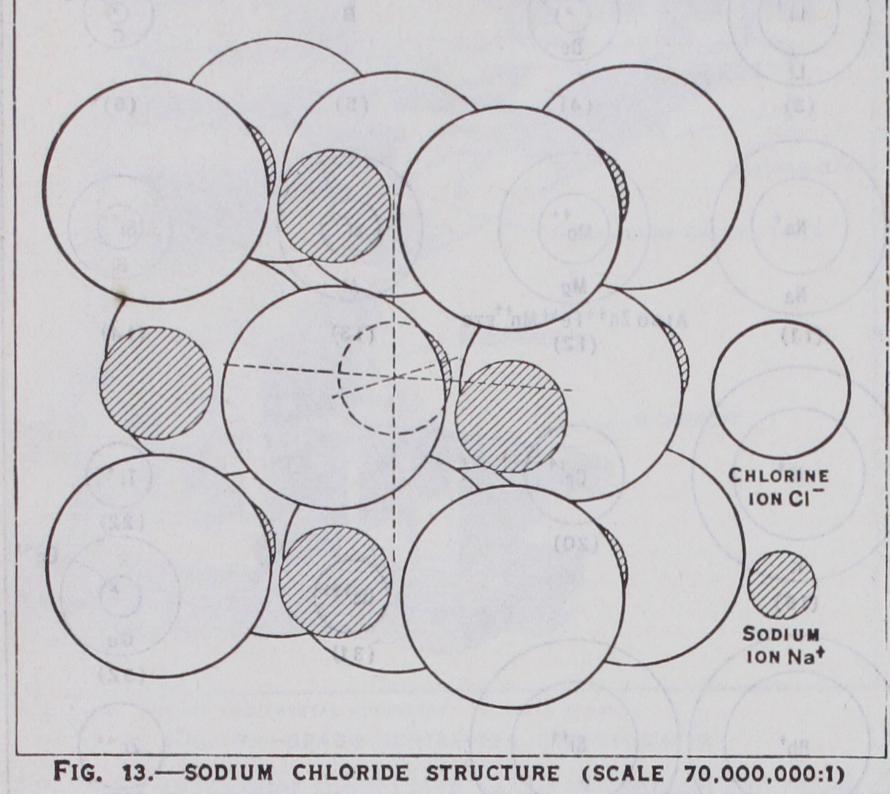
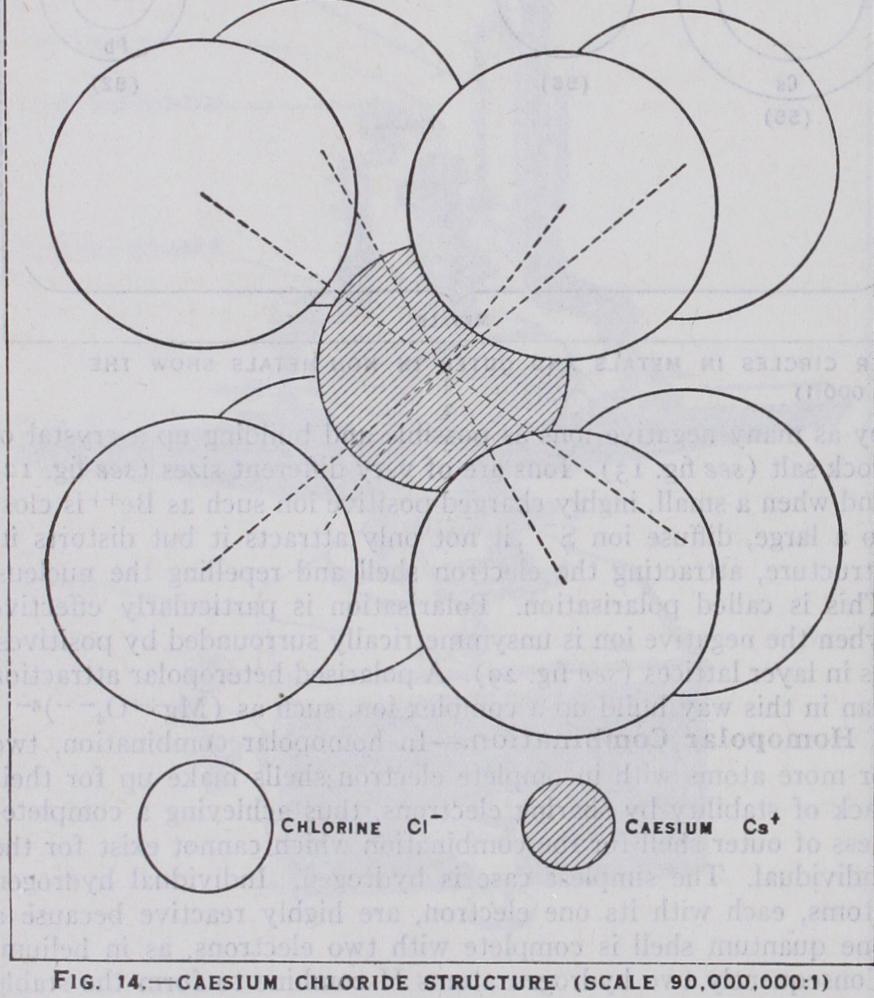
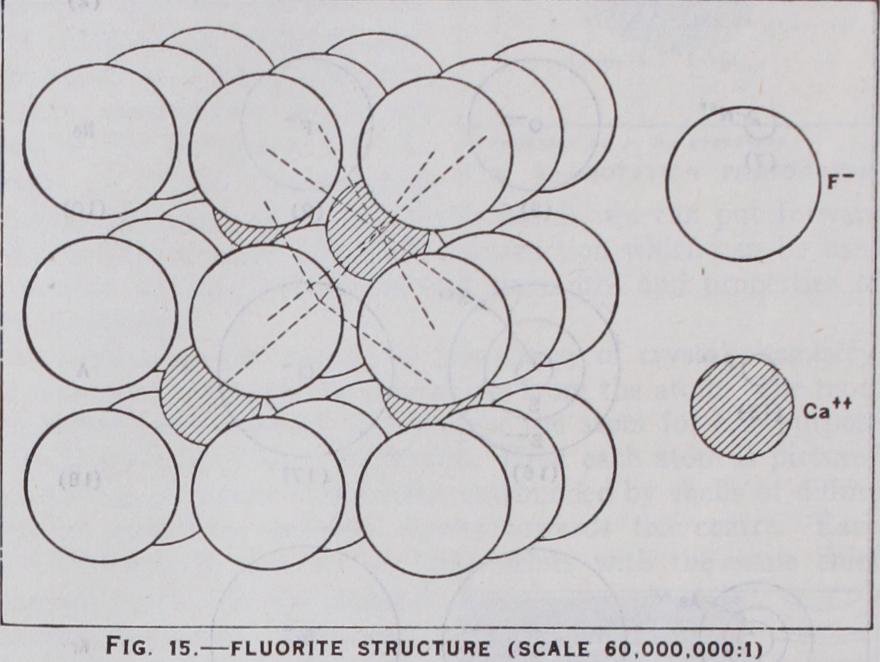
Two ions such as and will at tract each other with a force where r is the distance between them, and there will be an equilibrium when this force balances the repulsive force existing between their outer shells at close quarters. It is this repulsive force that gives the atoms their finite size. It is also electrical in origin but much more complex and expressible in such terms as — . In the presence, however, r + — of more and ions, it is easy to see that NaCl will become by as many negative ions as possible and building up a crystal of rock salt (see fig. 13) . Ions are of very different sizes (see fig. 12)and when a small, highly charged positive ion such as close to a large, diffuse ion it not only attracts it but distorts its structure, attracting the electron shell and repelling the nucleus. This is called polarisation. Polarisation is particularly effective when the negative ion is unsymmetrically surrounded by positives, as in layer lattices (see fig. 29). A polarised heteropolar attraction can in this way build up a complex ion, such as Homopolar Combination.—In homopolar combination, two or more atoms with incomplete electron shells make up for their lack of stability by sharing electrons, thus achieving a complete ness of outer shell for the combination which cannot exist for the individual. The simplest case is hydrogen. Individual hydrogen atoms, each with its one electron, are highly reactive because a one quantum shell is complete with two electrons, as in helium. Consequently two hydrogen atoms H combine to form the stable hydrogen molecule which spectrally and in many other ways resembles helium. In a similar way, by the sharing of a balanced pair of electrons, are formed the diatomic molecules F2, of the non-metallic elements. An atom can share electrons with more than one other atom. Oxygen sharing electrons with two ide O-C-O. The process, which can be extended indefinitely to form more and more complicated molecules, particularly those of organic chemistry (where the carbon atom possessing four two electron bonds [co-valencies in modern chemical terms] which bring its outer shell from four to eight electrons) enables long chains of atoms to be formed as in the paraffins, or rings as in ben zene derivatives. This reaches its ultimate limit when the linking is extended indefinitely in all directions and results, not in forming a molecule, but a crystal such as diamond (see fig. 23), which may be considered as one solid molecule. But homopolar bonds can also build up complex ions, such as the ammonium ion with its ten electrons, which has the same relation to the neutral mole cule CH4 as the sodium ion Na+ has to neon; or the series of negative ions S047P0417d Molecular Combination.—Between electrically neutral atoms and molecules which also have completed outer shells, there still exists a type of residual attraction which may be called molecular. In the simplest case of an inert gas atom, this residual attraction is only effective at very close distances and consequently, except at the lowest temperatures, the substance remains a gas. But at such temperatures it solidifies in a state of equilibrium between the attractive and repulsive forces, both probably due to distor tions in the electronic structure. Very similar is the attraction between non-polar molecules such as which also tend to have low melting points, but when a molecule is polar, that is, acts as an electric dipole or multipole, these poles attract others of oppo site sign, with forces approaching those of ionic crystals.