X-Rays and Crystal Structure
ions, atoms, ion, size, co-ordination, type, fig and electrons
Metallic Combination.—Metallic combination occurs when all the atoms tend easily to lose electrons. The positive ions thus formed cohere together, held by the electron gas, produced from the discarded electrons now no longer bound to particular atoms.
It is from such knowledge of the units of crystal structure, that is, atoms, simple and complex ions and molecules, and of the kind of forces holding them together, that we can see the physico chemical meanings of the crystal structures which are found by the quite independent means of X-rays. But the process is two sided. At the same time we learn from the structure much of the chemical and physical properties of atoms inaccessible by other methods.
Crystallised substances, that is to say, all solids with the ex ception of the glasses, may be divided into four main classes : the ionic, the adamantine, the molecular and the metallic; and three prominent intermediate classes : the silicates, the layer lattices and the metalloids. The leading properties of these classes are shown in Table I.
Ionic Crystals.
These have been more studied than any of the other classes because the simple Coulomb electrical forces holding them together lead to simpler structures, of which it is sometimes possible to give a quantitative explanation. The first law of forma tion for ionic crystals was stated by Goldschmidt as follows: "The crystal structure of a substance is determined by tile size and polarisation properties of its components, which may be atoms, ions or atomic groups." The size of an ion is, after its charge, its most important prop erty. It varies very greatly for different ions. In fig. 12 IS shown the size of a number of ions and atoms which illustrates their de pendence on atomic number and charge. It can be seen that the size of a positive ion in the same group increases with the atomic number, while in the same series for positive ions, it decreases markedly with increasing charge, which acts by tightening the whole structure. But in negative ions, the increased size due to the repulsion of the extra electrons is counteracted by the greater fields they find themselves in, and consequently doubly charged nega tive ions are never greater than and sometimes smaller than singly charged. The way in which the increased charge tightens the struc ture is shown by the fact that in KC1, where both ions have eight een electrons, the interatomic distance is 3.14 A; whereas in CaS, also with eighteen electrons each, but with quadruple electrostatic force, the corresponding distance has shrunk to 2.84 A.
The way in which atomic diameter influences structure can be seen from the simplest ionic structure of the type AX, with equal numbers of ions of opposite signs. The simplest of these is the structure of rock salt (see fig. 13), where sodium and chlorine ions occupy alternate corners of a cubic lattice. The co-ordination number is 6:6, that is, each sodium has six chlorine neighbours and vice versa. Actually, the chlorine ions are so large compared to the sodium that they form an octahedron that encloses it almost completely. Simple geometry shows that this can only be the case if RA (radius of positive ion ) : (radius of negative ion) <.73. This relation holds for all halides of the alkaline metals with the exception of the chloride, bromide and iodide of caesium where the ratios x are .91, •84 and -75 respectively. Now these last three are the only alkaline halides which do not belong to the sodium chloride type but to the caesium chloride type. (See fig. 14.) Here the co-ordination number is 8 :8 and there is, so to speak, more room for the larger caesium ion inside the cube of chlorine ions. Where is very small, the factor of polarisa tion comes in, and the structure becomes adamantine or molecular.
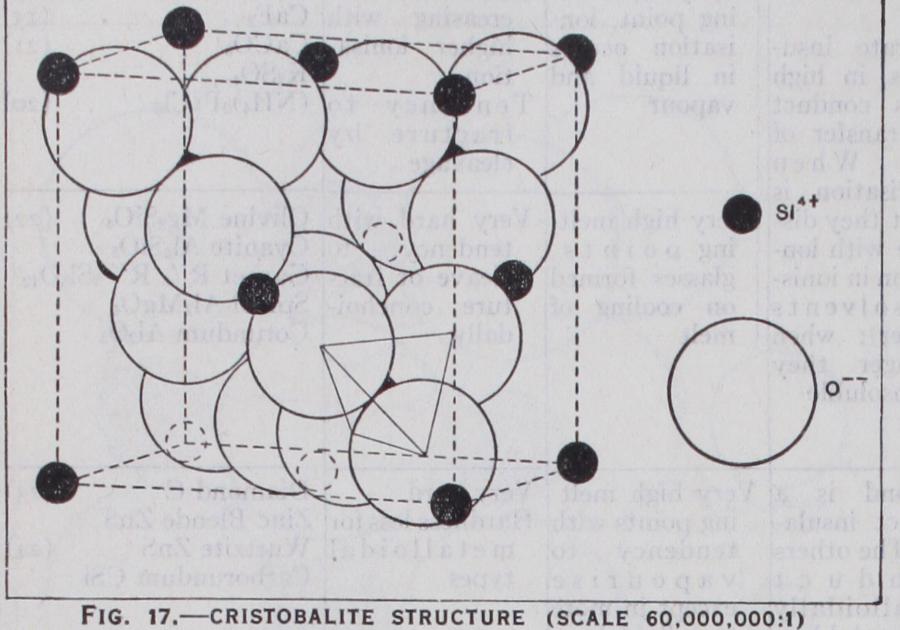
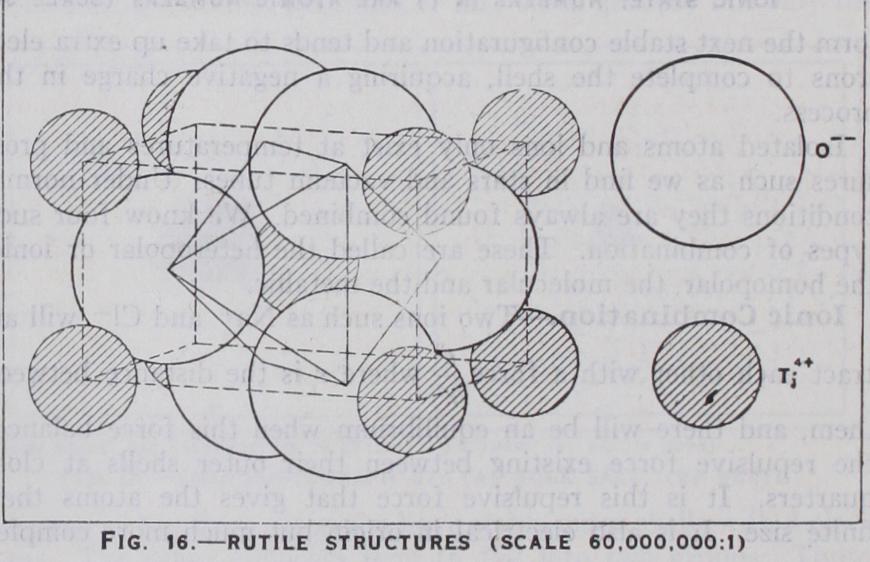
If we pass to the next simpler series, AX2, a similar situation occurs. Where is greater than .73, the structure is of the fluorite type. (See fig. i s.) Here the co-ordination number is 8 :4, the calcium ions being surrounded by a cube of eight fluorine ions, just as the caesium by the chlorines. A great number of compounds belong to this type, which includes the chlorides of the alkaline earths and the oxides of zirconium, thorium and uranium. If lies between •73 and -41, a structure is formed analogous to rock salt. This is the rutile structure. (See fig. 16.) Here the co-ordination number 6:3 cannot be satisfied in the cubic system and the octahedron of oxygen ions is placed on its side in a tetragonal structure. The two other forms of Ti02, anatase and brookite, are also built with the same co-ordination but with the octahedra distorted and differently placed. A great number of substances belong to the rutile structure. The fluorides of Mg., Mn., Co., Fe., Ni., Zn. and the dioxides of Mn., Mo., Sn., W. and Pb., as well as several others.